
| ·½·ØŅ» | ·½·Ø¶ž |
| ¢Ü½«25.00mLČÜŅŗÖĆÓŚÉÕ±ÖŠ£¬¼ÓČė¹żĮæµÄĻ”ŃĪĖį³ä·Ö½Į°č£» ¢Ż¼ÓČė¹żĮæBaCl2ČÜŅŗ£¬³ä·Ö½Į°č£¬Ź¹³ĮµķĶźČ«£» ¢Ž¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ³Įµķ”¢³ĘĮæµĆµ½bg¹ĢĢ壮 |
¢Ü½«25.00mLČÜŅŗÖĆӌ׶ŠĪĘæÖŠ£» ¢ŻÓĆ0.1mol/LµÄĖįŠŌKMnO4ČÜŅŗ½ųŠŠµĪ¶Ø£¬“ļµ½µĪ¶ØÖÕµćŹ±£¬ĻūŗÄ10.00mLČÜŅŗ£® |

| 1420b |
| 233a |
| 1420b |
| 233a |
| bg |
| 233g/mol |
| 10b |
| 233 |
| ||
| a |
| 1420b |
| 233a |
| 1420b |
| 233a |
| 1420b |
| 233a |


 ABCæ¼ĶõČ«ÓžķĻµĮŠ“š°ø
ABCæ¼ĶõČ«ÓžķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
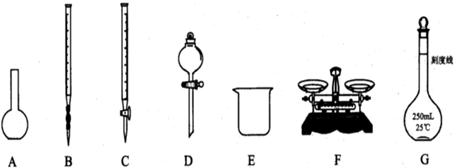
£Ø12·Ö£©ĪŖ²ā¶ØijNa2SO3ѳʷ£Øŗ¬ÉŁĮæNa2SO4ŌÓÖŹ£©µÄ“æ¶Č£¬ŹµŃéŹŅ°“ŅŌĻĀ²½Öč½ųŠŠ£»¢Ł ³ĘČ”agѳʷ£¬ÖĆÓŚÉÕ±ÖŠ£»¢Ś¼ÓČėŹŹĮæÕōĮóĖ®£¬Ź¹ŃłĘ·Čܽā£¬Č»ŗóÅäÖĘ³É250mLČÜŅŗ£»¢Ū×¼Č·ĮæČ”25.00mL²½Öč¢ŚÖŠÅäµĆµÄČÜŅŗ£»
Č»ŗó£¬æɲÉÓĆĻĀĮŠĮ½ÖÖ·½·Ø½ųŠŠ²ā¶Ø£ŗ
| ·½·ØŅ» | ·½·Ø¶ž |
| ¢Ü½«25.00mLČÜŅŗÖĆÓŚÉÕ±ÖŠ£¬¼ÓČė¹żĮæµÄĻ”ŃĪĖį³ä·Ö½Į°č£» ¢Ż¼ÓČė¹żĮæBaCl2ČÜŅŗ£¬³ä·Ö½Į°č£¬Ź¹³ĮµķĶźČ«£» ¢Ž¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ³Įµķ”¢³ĘĮæµĆµ½bg¹ĢĢ唣 | ¢Ü½«25.00mLČÜŅŗÖĆӌ׶ŠĪĘæÖŠ£» ¢ŻÓĆ0.1 mol/LµÄĖįŠŌKMnO4ČÜŅŗ½ųŠŠµĪ¶Ø£¬“ļµ½µĪ¶ØÖÕµćŹ±£¬ĻūŗÄ10.00mLČÜŅŗ”£ |
Ēėøł¾ŻÉĻŹöŹµŃ飬»Ų“š£ŗ
£Ø1£©ÉĻĶ¼ĖłŹ¾ŅĒĘ÷ÖŠ£¬±¾ŹµŃé²½Öč¢Ł¢Ś¢ŪÖŠ±ŲŠėÓƵĵÄŅĒĘ÷ŹĒEŗĶ £ØĢī×ÖÄø£©£»
£Ø2£©ŌŚ·½·ØŅ»¢ÜÖŠ¼ÓČė¹żĮæĻ”ŃĪĖįµÄÄæµÄŹĒ £»
£Ø3£©ŌŚ·½·ØŅ»²ā¶ØµĆµ½Na2SO3ѳʷµÄ“æ¶ČŹĒ £ØĮŠ³öĖćŹ½£¬æɲ»»Æ¼ņ£©£»
£Ø4£©ŌŚ·½·Ø¶žÖŠ£¬ŹĒ·ńŠčŅŖ¼ÓČėÖøŹ¾¼Į £ØĢī”°ŹĒ”±»ņ”°·ń”±£©£¬Ēė¼ņŹöĄķÓÉ £»
£Ø5£©ŌŚ·½·Ø¶žÖŠ“ļµ½µĪ¶ØÖÕµć¶ĮČ”Źż¾ŻŹ±£¬ø©ŹÓŅŗĆę£¬Ōņ²ā¶Ø½į¹ū
£ØĢī”°Ę«øß”±”¢”°Ę«µĶ”±”¢”°ĪŽÓ°Ļģ”±£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011½ģ¹óÖŻŹ”×ńŅåĖÄÖŠ×éĶÅ7Š£øßČżµŚŅ»“ĪĮŖæ¼£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗŹµŃéĢā
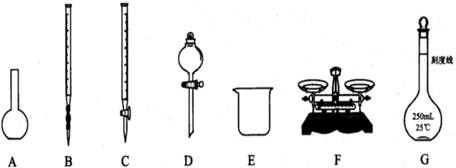
£Ø12·Ö£©ĪŖ²ā¶ØijNa2SO3ѳʷ£Øŗ¬ÉŁĮæNa2SO4ŌÓÖŹ£©µÄ“æ¶Č£¬ŹµŃéŹŅ°“ŅŌĻĀ²½Öč½ųŠŠ£»¢Ł³ĘČ”agѳʷ£¬ÖĆÓŚÉÕ±ÖŠ£»¢Ś¼ÓČėŹŹĮæÕōĮóĖ®£¬Ź¹ŃłĘ·Čܽā£¬Č»ŗóÅäÖĘ³É250mLČÜŅŗ£»¢Ū×¼Č·ĮæČ”25.00mL²½Öč¢ŚÖŠÅäµĆµÄČÜŅŗ£»
Č»ŗó£¬æɲÉÓĆĻĀĮŠĮ½ÖÖ·½·Ø½ųŠŠ²ā¶Ø£ŗ
| ·½·ØŅ» | ·½·Ø¶ž |
| ¢Ü½«25.00mLČÜŅŗÖĆÓŚÉÕ±ÖŠ£¬¼ÓČė¹żĮæµÄĻ”ŃĪĖį³ä·Ö½Į°č£» ¢Ż¼ÓČė¹żĮæBaCl2ČÜŅŗ£¬³ä·Ö½Į°č£¬Ź¹³ĮµķĶźČ«£» ¢Ž¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ³Įµķ”¢³ĘĮæµĆµ½bg¹ĢĢ唣 | ¢Ü½«25.00mLČÜŅŗÖĆӌ׶ŠĪĘæÖŠ£» ¢ŻÓĆ0.1 mol/LµÄĖįŠŌKMnO4ČÜŅŗ½ųŠŠµĪ¶Ø£¬“ļµ½µĪ¶ØÖÕµćŹ±£¬ĻūŗÄ10.00mLČÜŅŗ”£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğ¹óÖŻŹ”×éĶÅ7Š£øßČżµŚŅ»“ĪĮŖæ¼£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗŹµŃéĢā
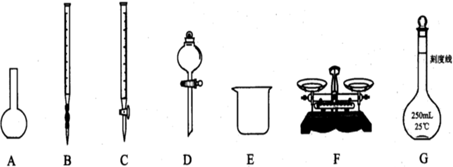
£Ø12·Ö£©ĪŖ²ā¶ØijNa2SO3ѳʷ£Øŗ¬ÉŁĮæNa2SO4ŌÓÖŹ£©µÄ“æ¶Č£¬ŹµŃéŹŅ°“ŅŌĻĀ²½Öč½ųŠŠ£»¢Ł ³ĘČ”agѳʷ£¬ÖĆÓŚÉÕ±ÖŠ£»¢Ś¼ÓČėŹŹĮæÕōĮóĖ®£¬Ź¹ŃłĘ·Čܽā£¬Č»ŗóÅäÖĘ³É250mLČÜŅŗ£»¢Ū×¼Č·ĮæČ”25.00mL²½Öč¢ŚÖŠÅäµĆµÄČÜŅŗ£»
Č»ŗó£¬æɲÉÓĆĻĀĮŠĮ½ÖÖ·½·Ø½ųŠŠ²ā¶Ø£ŗ
|
·½·ØŅ» |
·½·Ø¶ž |
|
¢Ü½«25.00mLČÜŅŗÖĆÓŚÉÕ±ÖŠ£¬¼ÓČė¹żĮæµÄĻ”ŃĪĖį³ä·Ö½Į°č£» ¢Ż¼ÓČė¹żĮæBaCl2ČÜŅŗ£¬³ä·Ö½Į°č£¬Ź¹³ĮµķĶźČ«£» ¢Ž¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ³Įµķ”¢³ĘĮæµĆµ½bg¹ĢĢ唣 |
¢Ü½«25.00mLČÜŅŗÖĆӌ׶ŠĪĘæÖŠ£» ¢ŻÓĆ0.1 mol/LµÄĖįŠŌKMnO4ČÜŅŗ½ųŠŠµĪ¶Ø£¬“ļµ½µĪ¶ØÖÕµćŹ±£¬ĻūŗÄ10.00mLČÜŅŗ”£ |
Ēėøł¾ŻÉĻŹöŹµŃ飬»Ų“š£ŗ
£Ø1£©ÉĻĶ¼ĖłŹ¾ŅĒĘ÷ÖŠ£¬±¾ŹµŃé²½Öč¢Ł¢Ś¢ŪÖŠ±ŲŠėÓƵĵÄŅĒĘ÷ŹĒEŗĶ £ØĢī×ÖÄø£©£»
£Ø2£©ŌŚ·½·ØŅ»¢ÜÖŠ¼ÓČė¹żĮæĻ”ŃĪĖįµÄÄæµÄŹĒ £»
£Ø3£©ŌŚ·½·ØŅ»²ā¶ØµĆµ½Na2SO3ѳʷµÄ“æ¶ČŹĒ £ØĮŠ³öĖćŹ½£¬æɲ»»Æ¼ņ£©£»
£Ø4£©ŌŚ·½·Ø¶žÖŠ£¬ŹĒ·ńŠčŅŖ¼ÓČėÖøŹ¾¼Į £ØĢī”°ŹĒ”±»ņ”°·ń”±£©£¬Ēė¼ņŹöĄķÓÉ £»
£Ø5£©ŌŚ·½·Ø¶žÖŠ“ļµ½µĪ¶ØÖÕµć¶ĮČ”Źż¾ŻŹ±£¬ø©ŹÓŅŗĆę£¬Ōņ²ā¶Ø½į¹ū
£ØĢī”°Ę«øß”±”¢”°Ę«µĶ”±”¢”°ĪŽÓ°Ļģ”±£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ·½·ØŅ» | ·½·Ø¶ž |
| ¢Ü½«25.00mLČÜŅŗÖĆÓŚÉÕ±ÖŠ£¬¼ÓČė¹żĮæµÄĻ”ŃĪĖį³ä·Ö½Į°č£» ¢Ż¼ÓČė¹żĮæBaCl2ČÜŅŗ£¬³ä·Ö½Į°č£¬Ź¹³ĮµķĶźČ«£» ¢Ž¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ³Įµķ”¢³ĘĮæµĆµ½bg¹ĢĢ壮 | ¢Ü½«25.00mLČÜŅŗÖĆӌ׶ŠĪĘæÖŠ£» ¢ŻÓĆ0.1mol/LµÄĖįŠŌKMnO4ČÜŅŗ½ųŠŠµĪ¶Ø£¬“ļµ½µĪ¶ØÖÕµćŹ±£¬ĻūŗÄ10.00mLČÜŅŗ£® |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com