
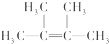 ��
��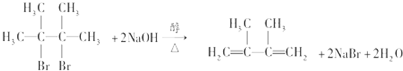 ��
�� ��
�� ���� ij��A����Է�������Ϊ84��������Cԭ�������Ŀ=$\frac{84}{12}$=7�����л������ʽΪC6H12��
��1�����������ĺ���������ͨʽ��֪CxHyOz���������ʵ���һ����������ֺ�������x+$\frac{y}{4}$-$\frac{z}{2}$����ȣ�����������������ȣ�������ȣ�
��2������AΪ������Ӧ����һ��̼̼˫�������������е�̼ԭ����ͬһƽ���ϣ��÷��ӵ�һ��ȡ����ֻ��һ�֣���AΪ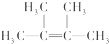 ��
��
A����������ˮ�����ӳɷ�Ӧ����BΪ��CH3��2CBrCBr��CH3��2��B��NaOH�Ĵ���Һ���ȿ��Եõ�D��D����������ԭ�ӣ�DΪCH2=C��CH3��-C��CH3��=CH2��B������NaOHˮ��Һ��ȫ��Ӧ���ɷ���ˮ�ⷴӦ���ɴ���
��3�����˴Ź���������ʾ����A�����鲻ͬ�ķ壬�������Ϊ3��2��1����Hԭ����Ŀ�ֱ�Ϊ6��4��2����AΪCH2=C��CH2CH3��2��
��4����A����ʹ��ˮ��ɫ������һ�ȴ���ֻ��һ�֣���AΪ�����飮
��� �⣺ij��A����Է�������Ϊ84��������Cԭ�������Ŀ=$\frac{84}{12}$=7�����л������ʽΪC6H12��
��1��1molC6H12�����=��6+3��mol=9mol��
���������ĺ���������ͨʽ��֪CxHyOz���������ʵ���һ����������ֺ�������x+$\frac{y}{4}$-$\frac{z}{2}$����ȣ�����������������ȣ�������ȣ�
a��1molC7H12O2 �����=��7+3-1��mol=9mol��
b��C6H14 �����=��6+$\frac{14}{4}$��mol=9.5mol��
c��C6H14O �����=��6+$\frac{14}{4}$-$\frac{1}{2}$��mol=9mol��
d��C7H14O3 �����=��7+$\frac{14}{4}$-$\frac{3}{2}$��mol=9mol��
����A�����������ϣ��������ʵ���һ�������ȼ������������������ȵ���b��
�ʴ�Ϊ��b��
��2������AΪ������Ӧ����һ��̼̼˫�������������е�̼ԭ����ͬһƽ���ϣ��÷��ӵ�һ��ȡ����ֻ��һ�֣���AΪ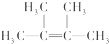 ��
��
A����������ˮ�����ӳɷ�Ӧ����BΪ��CH3��2CBrCBr��CH3��2��B��NaOH�Ĵ���Һ���ȿ��Եõ�D��D����������ԭ�ӣ�DΪCH2=C��CH3��-C��CH3��=CH2��
�������Ϸ�����֪AΪ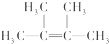 ���ʴ�Ϊ��
���ʴ�Ϊ��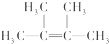 ��
��
��B����A��Br2��CCl4��Һ��Ӧ����B��B��NaOH�Ĵ���Һ���ȿɵõ�D��D����������ԭ�ӣ���BΪ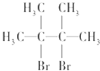 ��DΪ
��DΪ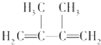 ������B�Ʊ�D�Ļ�ѧ����ʽΪ��
������B�Ʊ�D�Ļ�ѧ����ʽΪ��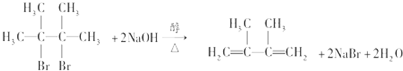 ��
��
�ʴ�Ϊ�� ��
��
��B������NaOHˮ��Һ��ȫ��Ӧ���ɷ���ˮ�ⷴӦ���ɴ���EΪ��CH3��2COHCOH��CH3��2����Ӧ�ķ���ʽΪ��CH3��2CBrCBr��CH3��2+2NaOH$��_{��}^{ˮ}$��CH3��2COHCOH��CH3��2+2NaBr���ʴ�Ϊ����CH3��2CBrCBr��CH3��2+2NaOH$��_{��}^{ˮ}$��CH3��2COHCOH��CH3��2+2NaBr��
��3�����˴Ź���������ʾ����A�����鲻ͬ�ķ壬�������Ϊ3��2��1����Hԭ����Ŀ�ֱ�Ϊ6��4��2����AΪCH2=C��CH2CH3��2������Ϊ��2-�һ�-1-��ϩ����CH3CH2CH=CHCH2CH3������Ϊ3-��ϩ���ʴ�Ϊ��2-�һ�-1-��ϩ��3-��ϩ��
��4����A����ʹ��ˮ��ɫ������һ�ȴ���ֻ��һ�֣���AΪ�����飬�ṹ��ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���� ���⿼���л����ƶϡ��л���ȼ�պ��������⡢��������ͬ���칹����д�ȣ�Ϊ��Ƶ���㣬������ѧ���ķ��������������Ŀ��飬��Ŀ�Ƚ��ۺϣ��ǶԻ���֪ʶ���ۺϿ��飬�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
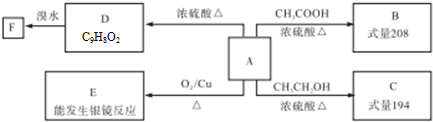
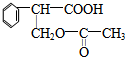 ��
�� ��
�� ��
��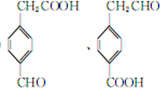 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�1.12L��������ԭ����Ϊ0.1NA | |
| B�� | 16 g O2��O3�Ļ�������У�������ԭ����Ϊ0.5NA | |
| C�� | 5.6 g ��������������ȼ�գ�ת�Ƶ�����Ϊ0.2 NA | |
| D�� | 9g D2O������������Ϊ4.5NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
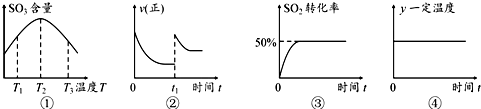
| A�� | ��ͼ�ٿ�֪���÷�Ӧ�ġ�H��0 | |
| B�� | ��ͼ���е�t1ʱ�������������������ѹǿ����ƽ������ | |
| C�� | ͼ����ʾ�����£�ƽ�ⳣ��Ϊ2 | |
| D�� | ͼ���е�y���Ա�ʾƽ�ⳣ�����ܶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʹ���Ը��������Һ��ɫ | |
| B�� | �����Ӿ���ƽ���������νṹ��12��ԭ����ͬһƽ���ϣ���λ�ϵ�4��ԭ����һ��ֱ���� | |
| C�� | ����ʹ��ˮ��ɫ���Ƿ�����ȡ����Ӧ | |
| D�� | ��������̼̼˫���ʲ��ܷ����ӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
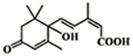 ���������ʻ���ʩ����S-�տ����Ƽ������Ա�֤�ʻ�ʢ����S-�տ��صķ��ӽṹ��ͼ�����й��ڸ÷��ӵ�˵����ȷ���ǣ�������
���������ʻ���ʩ����S-�տ����Ƽ������Ա�֤�ʻ�ʢ����S-�տ��صķ��ӽṹ��ͼ�����й��ڸ÷��ӵ�˵����ȷ���ǣ�������| A�� | 1mol��������������NaOH��Һ��Ӧ�������2mol NaOH | |
| B�� | ��������Cu�������������ܱ������� | |
| C�� | �������ܷ�����������ԭ��ȡ����Ӧ | |
| D�� | �ֱ���������Na��NaHCO3 ��Ӧ���ɵ���������ͬ״���������Ϊ2��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com