
[2012������]��14�֣���Ϣʱ�������Ĵ������������Ի��������˼������в��ij�����Ϊ����ѧ��̽��С�齫һ����������·������õ���70%Cu��25%Al��4%Fe������Au��Pt�Ƚ����Ļ�������Ƴ������Ʊ�����ͭ�������������·�ߣ�
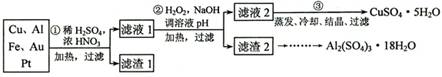
��ش��������⣺
��1���ڢٲ�Cu���ᷴӦ�����ӷ���ʽΪ_______________________________���õ�����1����Ҫ�ɷ�Ϊ___________________��
��2���ڢڲ���H2O2��������________________��ʹ��H2O2���ŵ���
������ҺpH��Ŀ����ʹ ���ɳ�����
��3���õڢ۲�����CuSO4��5H2O�Ʊ���ˮCuSO4�ķ����� ��
��4��������2��ȡAl2(SO4)3��18H2O ��̽��С����������ַ�����
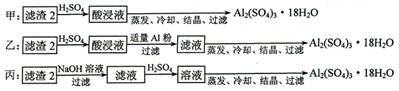
�������ַ����У�_______���������У�ԭ����_____________________________����ԭ�������ʽǶȿ��ǣ�________������������
��5��̽��С���õζ����ⶨCuSO4��5H2O ��Mr��250��������ȡa g�������100 mL��Һ��ÿ��ȡ20.00 mL�������������Ӻ���c mol��L��1 EDTA��H2Y2��������Һ�ζ����յ㣬ƽ������EDTA��ҺbmL���ζ���Ӧ���£�
Cu2+��H2Y2����CuY2����2H+
д������CuSO4��5H2O���������ı���ʽw�� ________________ �����в����ᵼ��CuSO4��5H2O�����IJⶨ���ƫ�ߵ���_____________��
a��δ������ƿ
b���ζ��յ�ʱ�ζ��ܼ����в�������
c��δ��������EDTA��Ӧ�ĸ�������
��14�֣���1��Cu��4H����2NO3��Cu2����2NO2����2H2O��
3Cu��8H����2NO3��3Cu2����2NO����4H2O�� Au��Pt
��2����Fe2������ΪFe3�������������ʣ��Ի�������Ⱦ�� Fe3����Al3��
��3��������ˮ
��4���ס����ò�Ʒ�к��н϶�Fe2(SO4)3���ʡ���
��5����100%�� c
����������1��Cu����ᷴӦ�����ӷ���ʽΪ��Cu��4H����2NO3��Cu2����2NO2����2H2O (������Ũ�Ƚϴ�ʱ�൱��Cu��ŨHNO3��Ӧ)��3Cu��8H����2NO3��3Cu2����2NO����4H2O(������Ũ�Ȳ��ܴ�ʱ�൱��Cu��ϡHNO3��Ӧ)��Au��Pt�������ᣬ��������ʽ�����˳�����
��2�������������Һ2������2�õ���Ʒ��֪����pH��Ŀ����ʹAl3����Fe3����ת��Ϊ������������2�С�
��3���ɵ�����ȡ��ˮ����ͭ��ֱ�Ӽ�����ˮ���ɡ�
��4��������2�к���Fe(OH)3����������û�г�ȥ���ʱ������Fe3����ʹ�Ƶþ����к��д�������Fe2(SO4)3���ʼ�������ȡ����������������Fe3����Ӧ����Ʒ�����ɵĺ������������࣬ԭ�������ʸߣ��������ĵ��ᡢ��࣬ԭ�������ʵ͡�
��5��a g��Ʒ�к���m(CuSO4��5H2O)��(10��3��b L��c mol/L)��250 g��mol��1������a g��Ʒ��CuSO4��5H2O����������Ϊ����100%��δ������ƿ�Բⶨ�����Ӱ�죬�ζ��յ�ʱ�ζ��ܼ����в�������ʹ��ȡ������EDTA��������٣��Ӷ����²ⶨ���ƫС��δ������EDTA��Ӧ�ĸ������ӽ�ʹ����EDTA�������²ⶨ���ƫ�ߡ�


 ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д� ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д� �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013���¿α���������¿���һ����ѧ�Ծ���B�������������� ���ͣ�ʵ����
[2012������]��14�֣���Ϣʱ�������Ĵ������������Ի��������˼������в��ij�����Ϊ����ѧ��̽��С�齫һ����������·������õ���70%Cu��25%Al��4%Fe������Au��Pt�Ƚ����Ļ�������Ƴ������Ʊ�����ͭ�������������·�ߣ�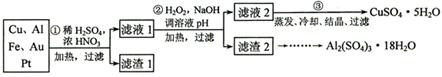
��ش��������⣺
��1���ڢٲ�Cu���ᷴӦ�����ӷ���ʽΪ_______________________________���õ�����1����Ҫ�ɷ�Ϊ___________________��
��2���ڢڲ���H2O2��������________________��ʹ��H2O2���ŵ���
������ҺpH��Ŀ����ʹ ���ɳ�����
��3���õڢ۲�����CuSO4��5H2O�Ʊ���ˮCuSO4�ķ����� ��
��4��������2��ȡAl2(SO4)3��18H2O ��̽��С����������ַ�����
�������ַ����У�_______���������У�ԭ����_____________________________����ԭ�������ʽǶȿ��ǣ�________������������
��5��̽��С���õζ����ⶨCuSO4��5H2O ��Mr��250��������ȡa g�������100 mL��Һ��ÿ��ȡ20.00 mL�������������Ӻ���cmol��L��1 EDTA��H2Y2��������Һ�ζ����յ㣬ƽ������EDTA��ҺbmL���ζ���Ӧ���£�
Cu2+��H2Y2����CuY2����2H+
д������CuSO4��5H2O���������ı���ʽw�� ________________ �����в����ᵼ��CuSO4��5H2O�����IJⶨ���ƫ�ߵ���_____________��
a��δ������ƿ
b���ζ��յ�ʱ�ζ��ܼ����в�������
c��δ��������EDTA��Ӧ�ĸ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���¿α���������¿���������ѧ�Ծ���A�����������棩 ���ͣ�ѡ����
[2012������] ���е��ʻ������ʵ�������ȷ����(����)
A��NaHSO4ˮ��Һ������
B��SiO2���ᡢ�������Ӧ
C��NO2����ˮʱ����������ԭ��Ӧ
D��Fe������Cl2��ȼ������FeCl2��FeCl3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���¿α���������¿���������ѧ�Ծ���A�������������� ���ͣ���ѡ��
[2012������] ���е��ʻ������ʵ�������ȷ����(����)
| A��NaHSO4ˮ��Һ������ |
| B��SiO2���ᡢ�������Ӧ |
| C��NO2����ˮʱ����������ԭ��Ӧ |
| D��Fe������Cl2��ȼ������FeCl2��FeCl3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com