
Ä³ÖŠŃ§»ÆѧŠĖȤŠ”×éĻėÖĘČ”±„ŗĶĀČĖ®£¬²¢½ųŠŠĀČĖ®µÄŠŌÖŹŹµŃ锣ĖūĆĒŹ¹ÓĆČēĶ¼×°ÖĆÖĘČ”½Ļ¶ąĮæµÄ±„ŗĶĀČĖ®£¬Ēė»Ų“š£ŗ
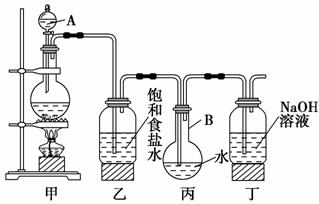
(1)Š“³öŅĒĘ÷µÄĆū³Ę£ŗA________£¬B________”£
(2)Š“³ö±ū”¢¶”×°ÖĆÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
±ū____________________________________________”£
¶”______________________________________________________”£
(3)ÓŠĶ¬Ń§Ģį³öČēĻĀøĽų½ØŅé£ŗ

¢ŁŌŚŅŅŗĶ±ūÖ®¼äŌö¼ÓÉĻĶ¼ÖŠµÄa×°ÖĆ£¬ÄćČĻĪŖÓŠĪŽ±ŲŅŖ________(Ģī”°ÓŠ”±»ņ”°ĪŽ”±)”£
¢ŚŌŚ±ūµÄ³¤µ¼¹ÜĻĀæŚ“¦£¬½ÓÉĻĶ¼ÖŠµÄb×°ÖĆ£¬æÉŅŌĢįøßĀČĘųµÄĪüŹÕŠ§¹ū”£ŌŅņŹĒ__________________________________________________________
_________________________________________________________ӣ
(4)ÓĆÖʵƵÄĀČĖ®·Ö±š½ųŠŠĻĀĮŠŹµŃé£ŗ¢ŁµĪČėĢ¼ĖįÄĘČÜŅŗÖŠ£¬ÓŠĘųĢåÉś³É£¬ĖµĆ÷ĀČĖ®ÖŠ·¢Éś·“Ó¦µÄĮ£×ÓŹĒ________________£»
¢ŚµĪČėAgNO3ČÜŅŗÖŠ£¬·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ_________________ ____________________________________________________________”£
(5)ÓƵĪ¹Ü½«ÖʵƵı„ŗĶĀČĖ®ĀżĀżµĪČėŗ¬·ÓĢŖµÄNaOHĻ”ČÜŅŗÖŠ”£µ±µĪµ½×īŗóŅ»µĪŹ±ŗģÉ«Ķ»Č»ĶŹČ„”£²śÉśÉĻŹöĻÖĻóµÄŌŅņæÉÄÜÓŠĮ½ÖÖ(¼ņŅŖĪÄ×ÖĖµĆ÷)£ŗ
¢ŁŹĒÓÉÓŚ_________________________________________________£»
¢ŚŹĒÓÉÓŚ___________________________________________________”£
ÓĆŹµŃéÖ¤Ć÷ŗģÉ«ĶŹČ„µÄŌŅņŹĒ¢Ł»ņÕߢŚµÄŹµŃé·½·ØŹĒ
___________________________________________________________________________________________________________________ӣ
(6)ĪŖĮĖĢįøßĀČĖ®ÖŠ“ĪĀČĖįµÄÅØ¶Č£¬ŌöĒæĀČĖ®µÄĘÆ°×ÄÜĮ¦£¬æÉĻņĀČĖ®ÖŠ¼ÓČėµÄŹŌ¼ĮŹĒ________”£
A£®CaCO3”” B£®Na2SO3””
C£®H2SO4”” D£®Ca(OH)2
½āĪö””(3)ŅŅŗĶ±ūÖ®¼äĪŽŠčŌö¼ÓøÉŌļ×°ÖĆ£¬ŅņĪŖ»¹ŅŖĶØČėĖ®ÖŠ”£
(5)NaOHÓė·ÓĢŖ×÷ÓĆ³ŹŗģÉ«£¬ŗģÉ«ĻūŹ§µÄŌŅņÓŠĮ½ÖÖ£ŗŅ»ŹĒNaOH±»ŃĪĖįÖŠŗĶĮĖ£»¶žŹĒ·ÓĢŖ±»HClOŃõ»ÆĮĖ”£µĪ¼ÓNaOHČÜŅŗ¼“æÉÅŠ¶Ļµ½µ×ŹĒÄÄÖÖŌŅņ”£
(6)ĀČĖ®ÖŠ“ęŌŚĘ½ŗāCl2£«H2OH£«£«Cl££«HClO£¬ÓÉÓŚ“ĪĀČĖįµÄĖįŠŌ±ČĢ¼ĖįČõ£¬CaCO3Ö»ÓėHCl·“Ó¦£¬²»ÓėHClO·“Ó¦£¬¶ųŹ¹Ę½ŗāÓŅŅĘ£¬HClOµÄÅضČŌö“ó”£Na2SO3”¢Ca(OH)2¾łÄÜÓėHClO·“Ó¦£¬¾”¹ÜĘ½ŗāÓŅŅĘ£¬µ«HClOµÄÅØ¶Č¼õŠ”£¬¼ÓČėH2SO4Ōö“óĮĖH£«ÅØ¶Č£¬Ź¹Ę½ŗā×óŅĘ£¬c(HClO)¼õŠ””£
“š°ø””(1)·ÖŅŗĀ©¶·””Ō²µ×ÉÕĘæ
(2)Cl2£«H2OH£«£«Cl££«HClO
Cl2£«2OH£===Cl££«ClO££«H2O
(3)¢ŁĪŽ””¢ŚæÉŌö“óCl2ÓėĖ®µÄ½Ó“„Ć껿
(4)¢ŁH£«””¢ŚAg£«£«Cl£===AgCl”ż
(5)ŃĪĖįÖŠŗĶĮĖĒāŃõ»ÆÄĘ””“ĪĀČĖį½«·ÓĢŖŃõ»ÆĮĖ””ĻņĶŹÉ«ŗóµÄČÜŅŗĄļŌŁµĪ¼ÓĒāŃõ»ÆÄĘČÜŅŗ£¬ČōČÜŅŗ±äŗģ£¬ĖµĆ÷ŹĒŌŅņ¢Ł£¬ČōČÜŅŗ²»±äŗģĖµĆ÷ŹĒŌŅņ¢Ś””(6)A


 Š”ѧ½Ģ²ÄĶźČ«½ā¶ĮĻµĮŠ“š°ø
Š”ѧ½Ģ²ÄĶźČ«½ā¶ĮĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖAŹĒŅ»ÖÖ·Ö×ÓĮæĪŖ28µÄĘųĢ¬Ģž£¬ĻÖŅŌAĪŖÖ÷ŅŖŌĮĻŗĻ³ÉŅ»ÖÖ¾ßÓŠ¹ūĻćĪ¶µÄĪļÖŹE£¬ĘäŗĻ³ÉĀ·ĻßČēĻĀĶ¼ĖłŹ¾”£
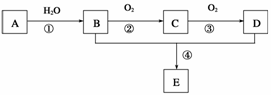
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)Š“³öAµÄ½į¹¹¼ņŹ½________________”£
(2)B”¢D·Ö×ÓÖŠµÄ¹ŁÄÜĶÅĆū³Ę·Ö±šŹĒ__________”¢______________”£
(3)ĪļÖŹBæÉŅŌ±»Ö±½ÓŃõ»ÆĪŖD£¬ŠčŅŖ¼ÓČėµÄŹŌ¼ĮŹĒ
________________________________________________________________________ӣ
(4)Š“³öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
¢Ł________________________________________________________________________£»
·“Ó¦ĄąŠĶ£ŗ____________”£
¢Ü________________________________________________________________________”£
·“Ó¦ĄąŠĶ£ŗ______________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ņ»¶ØĪĀ¶ČĻĀ£¬æÉÄę·“Ó¦3X(g)+Y(g)  2Z(g)“ļµ½ĻŽ¶ČµÄ±źÖ¾ŹĒ
2Z(g)“ļµ½ĻŽ¶ČµÄ±źÖ¾ŹĒ
A£®µ„Ī»Ź±¼äÄŚÉś³É3n mol X£¬Ķ¬Ź±ĻūŗÄn mol Y
B£®XµÄÉś³ÉĖŁĀŹÓėZµÄÉś³ÉĖŁĀŹĻąµČ
C£®X”¢Y”¢ZµÄÅضČĻąµČ
D£®X”¢Y”¢ZµÄ·Ö×ÓøöŹż±ČĪŖ3:1:2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĆĄ¹śÓīŗ½¾Ö”°·ļ»Ė”±ŗÅ»šŠĒµĒĀ½Ę÷µÄĻŌĪ¢”¢µē×Ó»Æѧ¼°“«µ¼·ÖĪöŅĒ¶ŌĮ½·ŻĶĮČĄŃł±¾µÄ·ÖĪö·¢ĻÖ£¬»šŠĒ±±¼«Ēų±ķ²ćĶĮČĄæÉÄÜŗ¬ÓŠøßĀČĖįŃĪ£¬æÉ““Ōģ²»ĄūÓŚČĪŗĪĒ±ŌŚÉśĆüµÄ¶ńĮÓ»·¾³”£ŌņĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ (””””)”£
A£®ŗ¬ÓŠøßĀČĖįŃĪµÄĶĮČĄ²»ĄūÓŚÉśĆü“ęŌŚÓėøßĀČĖįŃĪ¾ßÓŠ½ĻĒæµÄŃõ»ÆŠŌÓŠ¹Ų
B£®µ±ŌŖĖŲ“¦ÓŚ×īøß¼ŪĢ¬Ź±Ņ»¶Ø¾ßÓŠĒæŃõ»ÆŠŌ
C£®æÉŅŌæ¼ĀĒÓĆ¼ÓČėŃĒĢśŃĪµČ»¹ŌŠŌĪļÖŹµÄ·½·ØøÄÉĘÕāÖÖĶĮČĄ
D£®Ņ»¶ØĢõ¼žĻĀøßĀČĖįŃĪÄÜÓėÅØŃĪĖį·“Ӧɜ³ÉĀČĘų
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijĶ¬Ń§ĻņŅ»Ö§ŹŌ¹ÜÖŠ°“Ņ»¶ØµÄĖ³Šņ·Ö±š¼ÓČėĻĀĮŠ¼øÖÖĪļÖŹ(Ņ»ÖÖĪļÖŹÖ»¼ÓŅ»“Ī)£ŗ
a£®KIČÜŅŗ£»b.µķ·ŪČÜŅŗ£»c.NaOHČÜŅŗ£»d.Ļ”H2SO4£»e.ĀČĖ®”£·¢ĻÖČÜŅŗŃÕÉ«°“ČēĻĀĖ³Šņ±ä»Æ£ŗ¢ŁĪŽÉ«”ś¢Ś×Ų»ĘÉ«”ś¢ŪĄ¶É«”ś¢ÜĪŽÉ«”ś¢ŻĄ¶É«£¬¶Ō“Ė¹ż³Ģ½ųŠŠµÄ·ÖĪöÖŠ“ķĪóµÄŹĒ (””””)”£
A£®¼ÓČėŅŌÉĻŅ©Ę·µÄĖ³ŠņŹĒa”śe”śb”śc”śd
B£®¢Ū”ś¢Ü·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖI2£«2NaOH===NaI£«NaIO3£«H2O
C£®ČÜŅŗÓÉ×Ų»ĘÉ«±äĪŖĄ¶É«µÄŌŅņŹĒµķ·ŪČÜŅŗÓöµā±äĄ¶É«
D£®¢Ü”ś¢Ż·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ2I££«Cl2===I2£«2Cl£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ¢ń”¢¢ņŅņ¹ūĆčŹöÕżČ·ĒŅ¶žÕßÓŠ±ŲČ»ĮŖĻµµÄŹĒ (””””)”£
| ¢ń(ŌŅņ) | ¢ņ(½į¹ū) | |
| A | ľĢæ³ŹŗŚÉ« | ľĢæ³£ÓĆ×÷ÄÜŌ“ |
| B | Ņ»Ńõ»ÆĢ¼¾ßÓŠ»¹ŌŠŌ | ¹¤ŅµÉĻÓĆŅ»Ńõ»ÆĢ¼Ņ±Į¶½šŹō |
| C | Ģ¼ĖįÄĘŹōÓŚ¼īĄą | ÓĆČȵēæ¼īČÜŅŗĒåĻ“ÓĶĪŪĘ÷¾ß |
| D | ¶žŃõ»ÆĢ¼ŹĒĖįŠŌŃõ»ÆĪļ | ¶žŃõ»ÆĢ¼ÄÜÓėĖ®·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹č¼°Ęä»ÆŗĻĪļ¶ŌČĖĄąĻÖ“śĪÄĆ÷¾ßÓŠĢŲŹā¹±Ļ×”£Ēė»Ų“šĻĀĮŠÓŠ¹ŲĪŹĢā£ŗ
(1)¹čŌ×ӵĽį¹¹Ź¾ŅāĶ¼ĪŖ£ŗ________”£
(2)ĻĀĮŠĪļĘ·»ņÉč±øĖłÓĆ²ÄĮĻŹōÓŚ¹čĖįŃĪµÄŹĒ____________________________ _______________________________________”£
¢Ł³¤½ČżĻæĖ®Äą“ó°Ó””¢Ś¹āµ¼ĻĖĪ¬””¢ŪĢÕ“ÉŪįŪö
¢ÜĘÕĶز£Į§””¢Ż¹čĢ«ŃōÄܵē³Ų
A£®¢Ł¢Ś¢Ū”” B£®¢Ū¢Ü¢Ż””
C£®¢Ś¢Ū¢Ü”” D£®¢Ł¢Ū¢Ü
(3)ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ________”£
A£®ŌŚ“Ö¹čµÄÖĘȔ֊·¢Éś2C£«SiO2 2CO”ü£«Si£¬¹č±»»¹Ō£¬ĖłŅŌĢ¼µÄ»¹ŌŠŌ“óÓŚ¹čµÄ»¹ŌŠŌ
2CO”ü£«Si£¬¹č±»»¹Ō£¬ĖłŅŌĢ¼µÄ»¹ŌŠŌ“óÓŚ¹čµÄ»¹ŌŠŌ
B£®¹čĖįÄĘŹōÓŚŃĪ£¬²»ŹōÓŚ¼ī£¬ĖłŅŌ¹čĖįÄĘæÉŅŌ±£“ęŌŚÄ„æŚ²£Į§ČūŹŌ¼ĮĘæÖŠ
C£®ÓĆSiO2ÖĘČ”¹čĖį£¬Ó¦ĻČŹ¹¶žŃõ»Æ¹čÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦£¬Č»ŗóŌŁĶØČėCO2
D£®ÓÉNa2CO3£«SiO2 CO2”ü£«Na2SiO3æÉÖŖ¹čĖįµÄĖįŠŌ“óÓŚĢ¼Ėį
CO2”ü£«Na2SiO3æÉÖŖ¹čĖįµÄĖįŠŌ“óÓŚĢ¼Ėį
(4)³£ĪĀĻĀ£¬SiCl4ĪŖŅŗĢ¬£¬·ŠµćĪŖ57.6 ”ę£¬ŌŚæÕĘųÖŠĆ°°×Īķ”£Öʱøøß“æ¶Č¹čµÄÖŠ¼ä²śĪļSiCl4ÖŠČÜÓŠŅŗĢ¬ŌÓÖŹ£¬ČōŅŖµĆµ½øß“æ¶ČSiCl4£¬Ó¦²ÉÓƵķ½·ØŹĒ____________£»ÓĆ»Æѧ·½³ĢŹ½¼°±ŲŅŖµÄĪÄ×Ö½āŹĶSiCl4ŌŚæÕĘųÖŠĆ°°×ĪķµÄŌŅņ£ŗ______________________________________________________________
_______________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ¹ŲÓŚ½šŹōµÄŅ±Į¶·½·ØŠšŹö²»ÕżČ·µÄŹĒ (””””)”£
| ½šŹō | “ęŌŚŠĪŹ½ | Ņ±Į¶·½·Ø | |
| A | ½š | ÓĪĄėĢ¬ | ½š×Ó±Čɳ×ÓĆÜ¶Č“ó£¬ĄūÓĆĖ®Ļ“·ØÖ±½Ó·ÖĄė |
| B | Ņų | »ÆŗĻĢ¬ | ŅųµÄ½šŹōŠŌČõ£¬ÓĆ¼ÓČČAg2OµÄ·½·ØŅ±Į¶ |
| C | Ģś | »ÆŗĻĢ¬ | ĀĮµÄ½šŹōŠŌ±ČĢśĒ棬æÉÓĆĀĮČČ·ØĮ¶Ģś |
| D | ÄĘ | »ÆŗĻĢ¬ | ÄĘµÄ½šŹōŠŌĒ棬Ņ»°ć»¹Ō¼ĮŗÜÄŃ½«Ę仹Ō³öĄ“£¬ĖłŅŌÓƵē½ā±„ŗĶNaClČÜŅŗµÄ·½·ØŅ±Į¶ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹żŃõ»ÆÄĘ¾ßÓŠĒæŃõ»ÆŠŌ£¬ÓöľĢ攢ĀĮ·ŪµČ»¹ŌŠŌĪļÖŹŹ±æÉČ¼ÉÕ”£ĻĀĮŠÓŠ¹ŲĖµ·Ø²»ÕżČ·µÄŹĒ (””””)”£
A£®Na2O2ÓėCO2·“Ó¦Ź±£¬Na2O2ŹĒŃõ»Æ¼Į£¬CO2ŹĒ»¹Ō¼Į
B£®ČŪČŚ¹żŃõ»ÆÄĘŹ±²»æÉŹ¹ÓĆŹÆÓ¢ŪįŪö
C£®¹żŃõ»ÆÄĘÓėľĢ攢ĀĮ·Ū·“Ó¦Ź±£¬¹żŃõ»ÆÄĘ¾ł±ķĻÖ³öĒæŃõ»ÆŠŌ
D£®¹żŃõ»ÆÄĘÓė¶žŃõ»ÆĮņ·“Ó¦Ź±æÉÉś³ÉĮņĖįÄĘ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com