
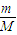 =
= £¬½įŗĻĪļÖŹµÄ·Ö×Ó×é³É¼ĘĖćĄė×ÓµÄĪļÖŹµÄĮæŗĶÅØ¶Č£»
£¬½įŗĻĪļÖŹµÄ·Ö×Ó×é³É¼ĘĖćĄė×ÓµÄĪļÖŹµÄĮæŗĶÅØ¶Č£» =
= £¬½įŗĻĦ¶ūÖŹĮæÓėĻą¶Ō·Ö×ÓÖŹĮæµÄ¹ŲĻµŅŌ¼°·Ö×ÓµÄ×é³ÉŌ×ÓµÄŌ×Ó½į¹¹¼ĘĖć£®
£¬½įŗĻĦ¶ūÖŹĮæÓėĻą¶Ō·Ö×ÓÖŹĮæµÄ¹ŲĻµŅŌ¼°·Ö×ÓµÄ×é³ÉŌ×ÓµÄŌ×Ó½į¹¹¼ĘĖć£®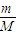 =
=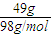 =0.5mol£¬
=0.5mol£¬ =
= =3mol£¬
=3mol£¬ ×n£ØH+£©=
×n£ØH+£©= ×3mol=1.5mol£¬
×3mol=1.5mol£¬ =
=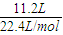 =0.5mol£¬¶ųÖŹĮæĪŖ17g£¬
=0.5mol£¬¶ųÖŹĮæĪŖ17g£¬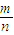 =
= =34g/mol£¬
=34g/mol£¬ æÉÖŖ£¬µČĪļÖŹµÄĮæµÄNH3ÓėH2SÖŹĮæ±ČµČÓŚĻą¶Ō·Ö×ÓÖŹĮæÖ®±Č£¬¼“17£ŗ34=1£ŗ2£¬
æÉÖŖ£¬µČĪļÖŹµÄĮæµÄNH3ÓėH2SÖŹĮæ±ČµČÓŚĻą¶Ō·Ö×ÓÖŹĮæÖ®±Č£¬¼“17£ŗ34=1£ŗ2£¬ =0.1mol£¬
=0.1mol£¬ =0.1mol£¬
=0.1mol£¬


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø1£©ĀČŌ×ӵĽį¹¹Ź¾ŅāĶ¼ĪŖ_____________________”£
£Ø2£©![]() Ī¢Į£ÖŠµÄÖŹ×ÓŹżŹĒ_______£¬ÖŠ×ÓŹżŹĒ_______£¬ŗĖĶāµē×ÓŹżŹĒ_______”£
Ī¢Į£ÖŠµÄÖŹ×ÓŹżŹĒ_______£¬ÖŠ×ÓŹżŹĒ_______£¬ŗĖĶāµē×ÓŹżŹĒ_______”£
£Ø3£©49g ĮņĖįµÄĪļÖŹµÄĮæĪŖ mol£¬ĘäĶźČ«µēĄė²śÉśH+µÄøöŹżĪŖ ”£
£Ø4£©V L Al2(SO4)3ČÜŅŗÖŠ£¬ŗ¬ÓŠa g Al3+£¬ŌņČÜŅŗÖŠAl3+µÄĪļÖŹµÄĮæÅØ¶Č £»
SO42- ĪļÖŹµÄĮæÅØ¶Č ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com