
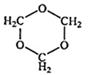
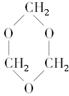
��֪��![]()
 ��R��R�䡢R�塪������H��
��R��R�䡢R�塪������H��
��1��B���ӽṹ��ֻ��һ���⡢һ������һ��̼����B�Ľṹ��ʽ��_____________��B��ͬ���칹�����������Ǿ������ƽṹ����___________________����д�ṹ��ʽ��
��2��F�Ľṹ��ʽ��_____________��PBT����__________���л��߷��ӻ����
��3����A��D����E�ķ�Ӧ����ʽΪ____________���䷴Ӧ����Ϊ____________________��
��4��E��ͬ���칹��C���ܷ���������Ӧ����ʹ��ˮ��ɫ����ˮ���Ҳ����̼ԭ�������ȣ���G��NaOH��Һ�з���ˮ�ⷴӦ�Ļ�ѧ����ʽ��__________________________________��
(1)
![]()
(2)HO��CH2��CH2��CH2��CH2��OH �����������
(3)HC��CH+2HCHO![]() HOCH
HOCH
(4)CH2=CH��COOCH3+NaOH![]() CH2=CH��COONa+CH3OH
CH2=CH��COONa+CH3OH
��������1����ͼ��֪CH3OH������ΪHCHO��HCHO���ɻ�״������B�Ľṹ��ʽΪ ��C3H6O3�������������ǵĽṹ��дΪ
��C3H6O3�������������ǵĽṹ��дΪ![]() ��
��
��2����AΪHCHO��FΪC4H10O2��F�����Ԫ����ۺϣ�˵��F��������OH��
��֪DΪHC��CH������Ϣ��֪HC��CH+2HCHO![]() HO��CH2C��C��CH2OH������F�ṹ��ʽΪHO��CH2��CH2��CH2��CH2��OH����PBTΪ�������л��߷��ӻ����
HO��CH2C��C��CH2OH������F�ṹ��ʽΪHO��CH2��CH2��CH2��CH2��OH����PBTΪ�������л��߷��ӻ����
��3���ɷ�Ӧ�ص��֪A+D![]() E�ķ�Ӧ����Ϊ�ӳɷ�Ӧ��
E�ķ�Ӧ����Ϊ�ӳɷ�Ӧ��
��4��E��ͬ���칹��G������������Ӧ��˵���ޡ�CHO����ʹ��ˮ��ɫ��˵�������ں��в����ͽṹ����ˮ��˵����������������ˮ������̼ԭ�������ȣ���֪��ṹΪCH2=CH��COOCH3��ˮ�ⷽ��ʽΪCH2=CH��COOCH3+NaOH![]() CH2=CH��COONa+CH3OH��
CH2=CH��COONa+CH3OH��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


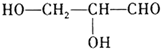
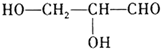
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
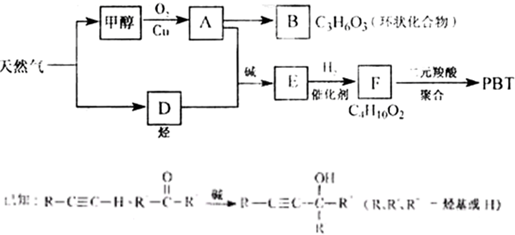
��08�����������16�֣���Ȼ�������������е�֧����ҵ֮һ.����Ȼ��Ϊԭ�Ͼ����з�Ӧ·�߿ɵù�������PBT.
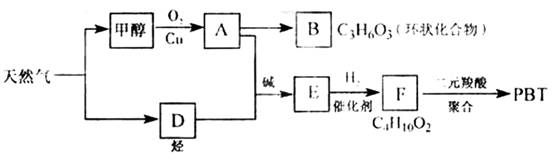
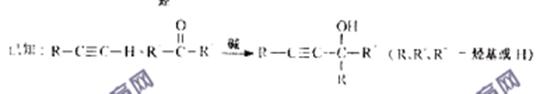
��1��B���ӽṹ��ֻ��һ���⡢һ������һ��̼����B�Ľṹ��ʽ�� ��B��ͬ���칹�����������Ǿ������ƽṹ���� .��д�ṹ��ʽ��
��2��F�Ľṹ��ʽ�� ��PBT���� ���л��߷��ӻ�����.
��3����A��D����E�ķ�Ӧ����ʽΪ ���䷴Ӧ����Ϊ .
��4��E��ͬ���칹��G���ܷ���������Ӧ����ʹ��ˮ��ɫ����ˮ���Ҳ����̼ԭ�������ȣ���G��NaOH��Һ�з���ˮ�ⷴӦ�Ļ�ѧ����ʽ�� .
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08�����������16�֣���Ȼ�������������е�֧����ҵ֮һ.����Ȼ��Ϊԭ�Ͼ����з�Ӧ·�߿ɵù�������PBT.
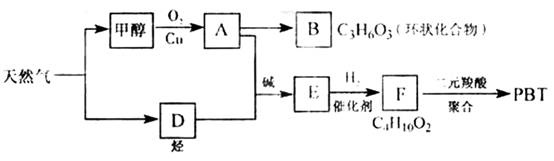
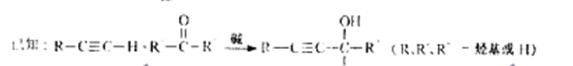
��1��B���ӽṹ��ֻ��һ���⡢һ������һ��̼����B�Ľṹ��ʽ�� ��B��ͬ���칹�����������Ǿ������ƽṹ���� .��д�ṹ��ʽ��
��2��F�Ľṹ��ʽ�� ��PBT���� ���л��߷��ӻ�����.
��3����A��D����E�ķ�Ӧ����ʽΪ ���䷴Ӧ����Ϊ .
��4��E��ͬ���칹��G���ܷ���������Ӧ����ʹ��ˮ��ɫ����ˮ���Ҳ����̼ԭ�������ȣ���G��NaOH��Һ�з���ˮ�ⷴӦ�Ļ�ѧ����ʽ�� .
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008����ͨ�ߵ�ѧУ����ͳһ�����������⻯ѧ���֣�������� ���ͣ��ƶ���
��16�֣���Ȼ�������������е�֧����ҵ֮һ������Ȼ��Ϊԭ�Ͼ����з�Ӧ·�߿ɵù�������PBT��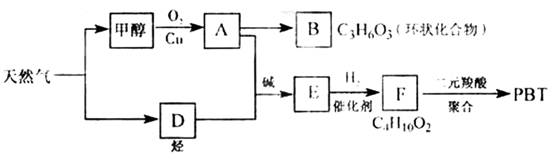
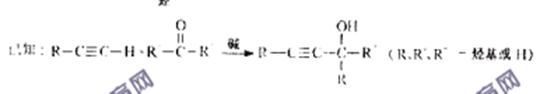
��1��B���ӽṹ��ֻ��һ���⡢һ������һ��̼����B�Ľṹ��ʽ�� ��B��ͬ���칹�����������Ǿ������ƽṹ���� ����д�ṹ��ʽ��
��2��F�Ľṹ��ʽ�� ��PBT���� ���л��߷��ӻ����
��3����A��D����E�ķ�Ӧ����ʽΪ ���䷴Ӧ����Ϊ ��
��4��E��ͬ���칹��G���ܷ���������Ӧ����ʹ��ˮ��ɫ����ˮ���Ҳ����̼ԭ�������ȣ���G��NaOH��Һ�з���ˮ�ⷴӦ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com