
��ij������Һ�У���Cl-֮����ܻ����е����ʵ����������������е�һ�ֻ���֣�SO32-��CO32-��SiO32-��I-��NO3-��SO42-���ڴ���Һ�м���������ᣬ�������ݣ���Һ��ɫ������Գ��壬����ԭ��Һ�е����������������3�֡��Իش��������⡣
��1��ԭ��Һ���Ƿ���SiO32-�� ����С���û�С������ж������� ���������ӷ���ʽ��ʾ��
��2�����ɵ�������һ���� �������е������� (����ĸ���)��
A����ɫ��ζ
B����ɫ�д̼�����ζ
C�����ڴ�����Ⱦ��
D��������ˮ
E���ܱ�NaOH��Һ����
��3��ԭ��Һ�п��ܺ��е��������� ��
��4����Һ�м��ٵ������� ��ԭ���� �������ӷ���ʽ��ʾ����
��1��û�У�1�֣���SiO32-+2H+=H2SiO3����1�֣�
��2��NO��1�֣���ACD��1�֣���
��3��SO42-��1�֣�
��4��SO32-��I-��NO3-����2�֣�
6I-+8H++2NO3-=3I2+2NO��+4H2O����2�֣�
3SO32-+2H++2NO3-=3SO42-+2NO��+H2O��2�֣�
��������
����������������������Һ�Գ����֪��SiO32����SiO32-+2H+=H2SiO3������Һ��ɫ���һ����I�� NO3��������Ӧ��6I����2NO3����8H��=3I2��2NO����4H2O�����������һ����NO�������������ʵ�������ȣ�����������Ӧ��NO3����ʣ�࣬��ˣ�ʹNO3��ȫ����Ӧ����Ҫ��SO32����3SO32-+2H++2NO3-=3SO42-+2NO��+H2O�������ʵ�����SO32����I�� ��NO3��ǡ����ȫ��Ӧ2I����2SO32-��2NO3����4H��=3I2��2NO����2SO42-��2H2O������3�����Ӽ��٣������CO32������Һ�м��ٵ�����Ӧ��SO32����I�� ��NO3����ԭ��Һ�п��ܺ��е���������SO42-��
���㣺�������Ӽ���Ͷ��������ƶϣ����ѡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
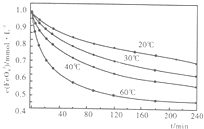 ��2010?����ģ�⣩������أ�K2FeO4����һ�ּ�������������������ɱ���������ȥ�ǡ���ɫ������Ϊһ������͡���Ч����ɫ�����Ķ��ˮ����������ʮ���������ҹ��Ը������������ˮ�����е�Ӧ�õ��о�Ҳ�������룬��ȡ�ÿ�ϲ�ɹ����Ƚ�������Ʊ������Ǵ�������������������KOH��Һ��ͨ������Cl2�Ʊ�������ر�����Һ���ٷִμ���KOH���壬�õ��������ǿ���Ա�����Һ�������������Σ��ϳɸ�����أ�
��2010?����ģ�⣩������أ�K2FeO4����һ�ּ�������������������ɱ���������ȥ�ǡ���ɫ������Ϊһ������͡���Ч����ɫ�����Ķ��ˮ����������ʮ���������ҹ��Ը������������ˮ�����е�Ӧ�õ��о�Ҳ�������룬��ȡ�ÿ�ϲ�ɹ����Ƚ�������Ʊ������Ǵ�������������������KOH��Һ��ͨ������Cl2�Ʊ�������ر�����Һ���ٷִμ���KOH���壬�õ��������ǿ���Ա�����Һ�������������Σ��ϳɸ�����أ�| �ŵ� |
| ��� |
| cV1 |
| 3V |
| cV1 |
| 3V |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| �� |
| ���� |
| �� |
| ���� |
| ŨH2SO4 |
| 170�� |
| ŨH2SO4 |
| 170�� |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�����и���5�¸߿�ģ�����ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
��Ԫ������Ȼ������Ҫ��������������Ҫ�ɷ�ΪAl2O3��������Fe2O3��FeO��SiO2���С���ҵ�����������Ʊ�����ij�ֻ�����Ĺ����������¡�
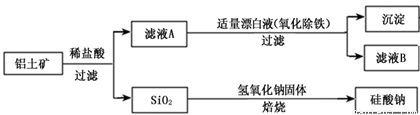
��1������ҺA�м���Ư��Һ��Ŀ��������������������ҺB�����ԡ�
�ٸù������漰ij������ԭ��Ӧ���£�����ɣ�
��Fe2++��ClO��+ =��Fe(OH)3��+��C1��+
�ڼ�����ҺB���Ƿ�����Ԫ�صķ���Ϊ�� ��ע���Լ�������
�۽���ҺB�е���Ԫ���Գ�����ʽ��������ѡ�õ�����Լ�Ϊ ������ţ���
a������������Һ b��������Һ c����ˮ d��������̼
������ҺB�Ʊ��Ȼ��������漰�IJ���Ϊ���ߵμ�Ũ���������Ũ������ȴ�ᾧ�� ����������ƣ���ϴ�ӡ�
��2����֪H2O2��һ�����ᣬ��ǿ������Һ����Ҫ��HO2����ʽ���ڡ�Ŀǰ�о��Ƚ����ŵ�Al��H2O2ȼ�ϵ�أ���ԭ������ͼ��ʾ������ܷ�Ӧ���£�2Al+3HO2��=2AlO2��+OH��+H2O

��������ӦʽΪ ��
��Al�缫�ױ�NaOH��Һ��ʴ�����Ǹõ��Ŀǰδ���ƹ�ʹ�õ�
ԭ��֮һ���缫����ʴ�����ӷ���ʽΪ ��
��3���ֲĶ������Է�ֹ�ֲĸ�ʴ���ڶ��������е��Һ���������Σ��ɷ�NaCl��KCl�����ʱ��Ԫ�غ���Ԫ����Ҫ��AlCl4����ʽ���ڣ����������Ȼ�����Һ��ԭ���� ��
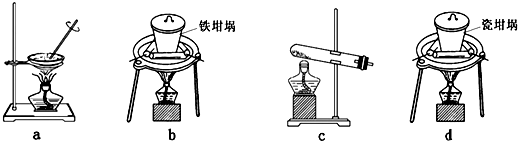
��4��SiO2��NaOH�����Ʊ������ƣ��ɲ��õ�װ��Ϊ ������ţ���
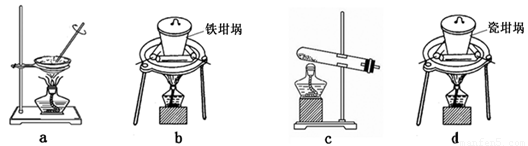
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com