
��12�֣�����ѧ����ѧ�뼼����
�����ǹ�ҵ��������Ϊ��Ҫ�IJ�Ʒ֮һ���ڻ�ѧ��ҵ�ĺܶ�����Ҫ�õ�Ũ���ᡣ
��1�������Ṥҵ�����У��ҹ����û�����Ϊԭ������SO2����Ӧ�Ļ�ѧ����ʽΪ ���÷�Ӧ�� �н��У����豸���ƣ���
��2��Ϊ��������SO2ת��ΪSO3�����ܳ���������ܣ������˶����������Ƚ������� �����豸���ƣ��н��з�Ӧ������ͼ��ʾ��װ���У�C�������������� ��SO3���� �����豸���ƣ���____ ���գ��õ�Ũ����������ᡣ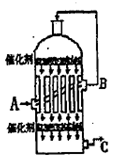
��3��ʵ����SO2��Ӧ����SO3��ת�������¶ȡ�ѹǿ�йأ�������±���Ϣ����Ϲ�ҵ����ʵ�ʣ�ѡ������ʵ����������� ��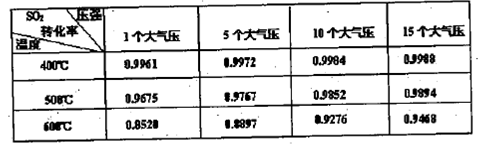
��4���������ų���β����SO2�ĺ����������500 L/L����Ҫ���Դ���������ų�����������֮һ���ð�ˮϴ�����������÷���ʽ��ʾ��ˮϴ���������ų���β���Ļ�ѧ����ʽ
L/L����Ҫ���Դ���������ų�����������֮һ���ð�ˮϴ�����������÷���ʽ��ʾ��ˮϴ���������ų���β���Ļ�ѧ����ʽ
��
��12�֣�
��1��4FeS2+11O2  2Fe2O3+8SO2��2�֣� ����¯��1�֣�
2Fe2O3+8SO2��2�֣� ����¯��1�֣�
��2���Ӵ��ң�1�֣�SO2��SO3��O2��2�֣���������1�֣�98.3%��H2SO4��1�֣�
��3��400��,1��������ѹ��2�֣�
��4��2NH3?H2O+SO2=(NH4)2SO3+H2O��2�֣�
���������������1�����������Ҫ�ɷ�Ϊ4FeS2������ʱ��O2��Ӧ����Fe2O3��SO2����ѧ����ʽΪ��4FeS2+11O2  2Fe2O3+8SO2�����������յ��豸�Ƿ���¯��
2Fe2O3+8SO2�����������յ��豸�Ƿ���¯��
��2��SO2ת��ΪSO3�ڽӴ����н��У�SO2��O2�ķ�ӦΪ���淴Ӧ������C�������������У�SO2��SO3��O2��Ϊ�˷�ֹ�������γɣ���98.3%��H2SO4����SO3������SO3���豸����������
��3����ΪSO2��O2�ķ�Ӧ�Ƿ��ȷ�Ӧ������ѡ����¶�Ϊ400��,��1��������ѹʱSO2��ת�����Ѿ��ܴ�������ѹǿ��SO2��ת������߲��������������ɱ�������ѡ��1��������ѹ��
��4��SO2Ϊ�����������Ӧ�����κ�ˮ����ѧ����ʽΪ��2NH3?H2O+SO2=(NH4)2SO3+H2O
���㣺���⿼������Ĺ�ҵ�Ʒ�����ѧ����ʽ����д��


 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ijЩ��ѧ֪ʶ�������ʾ�����ֳ�����ֱ�ۡ������ǵ��ص㡣�����������ʾ����������
| A��Cl2��CH4ȡ����Ӧ��IJ�� |  |
| B�����ռ�Һ��ͨ��SO2��IJ�� |  |
| C������ϡ���ᷴӦ�� |  |
| D����AlCl3��Һ�еμ�NaOH��Һ����Ԫ�صĴ�����ʽ�� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
1��92gCuƬ��һ������ŨHNO3���ã����ռ���NO2��NO���干1��12Lʱ����״����������ͭǡ��ȫ�����á���
��1����Ӧ������HNO3 mol��ת�Ƶ��� mol
��2������ˮ���ռ����ɵ�����,�����������Ϊ L (��״��)
��3�����ռ�����������ͨ�� mL O2(��״��)����ʹˮ�պó�����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��11�� ijУ��ѧ��ȤС���ͬѧ�������ε����ȷֽ����̽�����������������װ�÷ֱ������Ca��NO3��2��Cu(NO3)2��AgNO3���ֹ��塣�����ȼ��г�װ��δ������
��1����ͬѧ���ȵ���Ca��NO3��2�����ȹ��̷��֣�װ�â��в��� ���ݣ�����ʯ����Һ��ѹ��װ�â��У��ô����ǵ�ľ��������е����壬ľ����ȼ������װ�â���ʣ��Ĺ����֪��ʣ������к���NԪ�أ����ԣ�3�ۡ���д��Ca��NO3��2���ȷֽ�����ɲ���Ļ�ѧʽ�� �� ��
��2����ͬѧ���ȵ���Cu(NO3)2�����ȹ��̷��֣�װ�â���Ҳ�����ݲ��������������Ĺ�������ʧ��ʯ����Һ��Ϊ��ɫ��Һ�弸������ѹ��װ�â��С�װ�â��еĹ�����Ϊ��ɫ����д��Cu(NO3)2���ȷֽ�Ļ�ѧ����ʽ�� ��
��3����ͬѧ���ȵ���AgNO3�����ȹ��̷��֣�װ�â���Ҳ�����ݲ��������������Ĺ��������ݲ�����ʧ��ʣ�������Ҳ��ʹ�����ǵ�ľ����ȼ��ʯ����ҺҲ��Ϊ��ɫ��������Һ�屻ѹ��װ�â��С�װ�â��еĹ�����Ϊ��ɫ����ͬѧ�ݴ�д����AgNO3���ȷֽ���ܵ����ֻ�ѧ����ʽ��
����4AgNO3  2Ag2O��4NO2����O2�� ����2AgNO3
2Ag2O��4NO2����O2�� ����2AgNO3 2Ag��2NO2����O2����
2Ag��2NO2����O2����
������ȷ���� ����˵�����ɣ� ��
�������һ����ʵ��֤����Ľ�������ȷ�ģ� ��
��4��������3��ʵ��Ľ���������Ʋ����������ȷֽ�Ĺ��ɣ� ��
��5����������ͬѧ����������ag��������������ȫ�ֽ��ȡ��Ͳ���Ϊbml�����������ķֽ��ʣ�____________�����������������ϵ���ɲ��û���С����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�о�С��ģ�ҵ������ƺ����ˮ���ⶨ������Ч�ʣ�������ͼ��ʾװ�ý���ʵ�顣��CN����Ũ��Ϊ0.2 mol��L��1�ĺ����ˮ100 mL��100 mL NaClO��Һ������������װ�â�������ƿ�У���ַ�Ӧ����Һ©������������100 mLϡH2SO4���رջ�����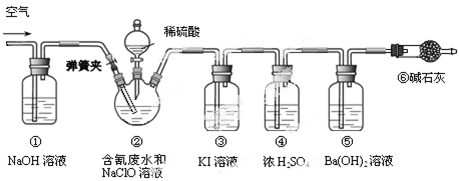
��֪װ�â��з�������Ҫ��Ӧ����Ϊ��
CN��+ ClO��=CNO��+ Cl�� 2CNO��+2H+ +3C1O��=N2��+2CO2��+3C1��+H2O
��1���ٺ͢������� ��
��2��װ�â��У���������װ�â۳�ȥ�����ʵ����ӷ���ʽΪ ��
��3����Ӧ��������ͨ�������Ŀ���� ��
��4��Ϊ�����ʵ���к����ˮ�������İٷ��ʣ���Ҫ�ⶨ ��������
��5����֪CN-�Ĵ���Ч�ʿɸߴ�90%��������CO2�ڱ�״���µ����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ǵؿ��к�����Ϊ�ḻ�ķǽ���Ԫ��,��Ҫ��������ˮ����������Ca3(PO4)2����ʽ���ڡ����ĵ��ʺͻ������ڹ�ũҵ������������Ҫ��Ӧ�á�
��1������(P4)����Ca3(PO4)2����̿��SiO2��һ�������·�Ӧ��á�����Ȼ�ѧ����ʽ����:
��2Ca3(PO4)2(s)+10C(s) 6CaO(s)+P4(s)+10CO(g����H1="+3" 359��26 kJ��mol-1
6CaO(s)+P4(s)+10CO(g����H1="+3" 359��26 kJ��mol-1
��CaO(s)+SiO2(s) CaSiO3(s�� ��H2=-89��61 kJ��mol-1
CaSiO3(s�� ��H2=-89��61 kJ��mol-1
2Ca3(PO4)2(s)+6SiO2(s)+10C(s) 6CaSiO3(s)+P4(s)+10CO(g����H3��H3= kJ��mol-1��
6CaSiO3(s)+P4(s)+10CO(g����H3��H3= kJ��mol-1��
��2�������ж������CuSO4��Һ�ⶾ,�ⶾԭ���������л�ѧ����ʽ��ʾ: 11P4+60CuSO4 +96H2O 20Cu3P +24H3PO4+60H2SO4 6 mol CuSO4�������������ʵ����� ��
20Cu3P +24H3PO4+60H2SO4 6 mol CuSO4�������������ʵ����� ��
��3������Ҫ������NaH2PO4��Na2HPO4��Na3PO4��ͨ��H3PO4��NaOH��Һ��Ӧ���,�������ֵķֲ�����(ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ���)��pH�Ĺ�ϵ��ͼ��ʾ��
��Ϊ��þ����ܴ���NaH2PO4,pHӦ�������� pH=8ʱ,��Һ����Ҫ��������Ũ�ȴ�С��ϵΪ ��
��Na2HPO4��Һ�Լ���,������Һ�м���������CaCl2��Һ,��Һ��������,��ԭ���� (д���ӷ���ʽ)��
��4���Ļ�������������( )�뼾���Ĵ�(
)�뼾���Ĵ�( )�����ʵ���֮��2��1��Ӧʱ,�ɻ��һ��������ȼ���м���X,���ͷų�һ���������塣�����Ĵ���X�ĺ˴Ź�����������ͼ��ʾ��
)�����ʵ���֮��2��1��Ӧʱ,�ɻ��һ��������ȼ���м���X,���ͷų�һ���������塣�����Ĵ���X�ĺ˴Ź�����������ͼ��ʾ��
������������ (�ѧʽ)
��X�Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼÿһ�����е���ĸ����һ�ַ�Ӧ������������J�Ǻ�����Ԫ��A�İ�ɫ��״������IΪNaCl��Һ��D�ǵ���ɫ���嵥�ʡ�����д���пհס�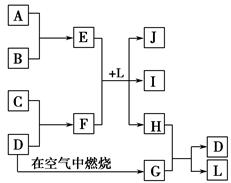
(1)��ͼ���������������ڷǵ���ʵ����ʵĻ�ѧʽ�� ��
(2)�õ���ʽ��ʾ��H���γɹ��� ��
(3)��E��ˮ��Һ���ɲ����յõ��Ĺ������ʵĻ�ѧʽΪ ��
(4)F��ˮ��Һ�и�����Ũ���ɴ�С��˳��Ϊ ��
(5)F��ˮ��Һ�Լ��Ե�ԭ�� (�����ӷ���ʽ��ʾ)��
(6)E��F��L�з�Ӧ�����ӷ���ʽΪ ��
(7)H��G֮�䷴Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�Ͽ���ͭм��Ũ����Ϊԭ����ȡ����ͭ����ʵ�������У��Ȱ�ͭм�ڿ��������գ��ٸ��õ����ˮϡ�͵�Ũ���ᷴӦ����ȡ����ͭ����ش��������⣺
(1)��������ͭмֱ�������ᷴӦ����ȡ����ͭ��ԭ���� ��
(2)Ũ�����õ����ˮϡ�͵�Ŀ���� ��
(3)Ҫ�õ�����ͭ���壬Ӧѡ�� ��
(4)��Ӧ��������ֳ� �ԡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
һЩ������ѧ��Ӧ������ʽ��ʾ A + B �� C+D+H2O ����AΪ���� (��Ҫʱ�ɼ���)����������������ʾ�ش��������⣺
��1����A��C��D��������Ԫ�أ���A��B��Һ��Ӧ�����ӷ���ʽΪ�� ��
��2����C��D��Ϊ��������һ��Ϊ����ɫ����B�� ��
��3����C��D��Ϊ�����Ҷ���ʹ����ʯ��ˮ����ǣ���A��B��Ӧ�Ļ�ѧ����ʽΪ�� ��
���ϡ��B��Ũ��Һ ��
(4) ��AΪ�Ϻ�ɫ�Ĺ��壬DΪ��ɫ��ζ�����壬��A��B��Һ��Ӧ�����ӷ���ʽΪ�� ����������״����4��48L��D���壬��ԭ��B�����ʵ����� mol��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com