
| �� �� | Fe��OH��2 | Cu��OH��2 | Fe��OH��3 |
| ��ȫ����ʱ��pH��Χ | ��9.6 | ��6.4 | 3��4 |

 CuSO4��Һ
CuSO4��Һ CuSO4?5H2O��CuO
CuSO4?5H2O��CuO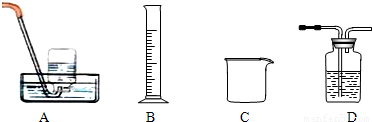


 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �� |
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | Cu��OH��2 | Fe��OH��2 | Fe��OH��3 |
| ��ʼ����pH | 6.0 | 7.5 | 1.4 |
| ������ȫpH | 13 | 14 | 3.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �� �� | Fe��OH��2 | Cu��OH��2 | Fe��OH��3 |
| ��ȫ����ʱ��pH��Χ | ��9.6 | ��6.4 | 3��4 |
| �� |
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)�Ʊ�����ͭ
![]() CuSO4��5H2O����CuO
CuSO4��5H2O����CuO
�ٲ�����Ŀ���dz����������ʡ�������___________________________________________��
�ڲ�����Ŀ���dz����������ǣ��μ�H2O2��Һ���Լ��ȣ�Fe2+ת����ȫ����������Cu2(OH)2CO3��ĩ�����裬�Կ�����ҺpH=3.5���������һ��ʱ�䣬���ˣ���ϡ�����ữ��Һ��pH=1��������ҺpH=3.5��ԭ����_________��
�۲�����Ŀ���ǵõ�CuSO4��5H2O���壬������__________________��ˮԡ���Ⱥ�ɡ�ˮԡ���ȵ��ص���___________________________________________________________��
(2)̽������ͭ����
��ȡA��B��֧�Թܣ���A���ȼ�������CuO��ĩ���ٷֱ���A��B�м���������3%H2O2��Һ��ֻ�۲쵽A���д������ݣ�������____________________________________________��
��Ϊ̽���Թ�A�з�Ӧ�����ʣ��ռ����岢�ⶨ����������ʵ��������______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ�����еڶ�ʮ��ѧ�����߿��캽���ԣ�������ѧ�Ծ����������� ���ͣ������
����ͭ�ж�����;��������������ɫ��������������ȡ�Ϊ��ô���������ͭ��ijͬѧ�ù�ҵ����ͭ(����������������)��������ʵ�飺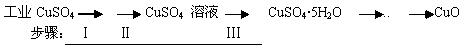
���������ϵ�֪������Һ��ͨ��������Һ������Զ�ʹCu2+��Fe2+��Fe3+�ֱ����ɳ�����pH���£�
| ���� | Cu(OH)2 | Fe(OH)2 | Fe(OH)3 |
| ��ʼ����pH | 6.0 | 7.5 | 1.4 |
| ������ȫpH | 13 | 14 | 3.7 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com