
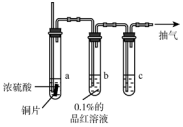
 ijʵ��С��ͬѧΪ��̽��ͭ��Ũ����ķ�Ӧ���Խ̲��е�װ�ý��мĽ����ڴ��Թ�a�м���һ�����������ܣ���ͼ��ͼ�мг������ͼ�������û�л�������
ijʵ��С��ͬѧΪ��̽��ͭ��Ũ����ķ�Ӧ���Խ̲��е�װ�ý��мĽ����ڴ��Թ�a�м���һ�����������ܣ���ͼ��ͼ�мг������ͼ�������û�л����������� ��1��ͭ��Ũ������ȷ�Ӧ���ɶ����������Ư�����ã�
��2��������������Ⱦ�����壬��������������Ա�����������Һ���գ�
��3��Ũ�����ͭ�ڼ������������ɶ�����������ͭ��ĩ�˳���ʹ��Ⱦ�������������ȫ��������������Һ���գ�a�г���������Ϊ��ѹǿƽ����ڳ������У�
��4�������ݲ����ɫ����Ϊ����ͭ����ͭ��Ũ���ᷴӦ��������ͭ�����������ˮ������ԭ���غ�����غ���ƽд����
�ں�ɫ�����CuO����ܺ��к�ɫ�Ĺ���Cu2S��CuS������������ԭ��ӦԪ�ػ��ϼ����������жϲ��
��5���������Һ�з������ϴ�Ӻ���Ҫ������أ�������Ҫ���Ƶ����غ�Ż������
��6�����ü�ֵ������ʵ�����������������������жϣ�
��� �⣺��1��Ũ�����ڼ��������±�ͭ��ԭΪ�����������壬�����������Ư�����ã���ʹƷ����Һ��ɫ���ʴ�Ϊ��Ʒ����Һ��ɫ��
��2���Թ�c��β������װ�ã�������������Ⱦ�����壬�������������Ҫ������������Һ���գ��ʴ�Ϊ��NaOH��Һ��
��3��Ũ�����ͭ�ڼ������������ɶ�����������ͭ������ʽΪCu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��װ���л���ʣ��Ķ����������壬�����ŷŵ������У���Ӧ���������Թ�c��ĩ�˳�����Ϊ�����ɵĶ�������ȫ�������գ����Թ�a�г��������ܵ������DZ���ѹǿ���ڳ������У�
�ʴ�Ϊ��ʹ���ɵĶ���������ȫ�����գ�ʹ�ó��������У�Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
��4���ټ�ͬѧ���룺��ɫ������δ���ܽ�����Ĵ�����CuO����ѧ����ʽ��ʾΪCu+H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuO+SO2��+H2O��
�ʴ�Ϊ��CuO+SO2��+H2O��
����ͬѧ��Ϊ��ɫ�����CuO����ܺ���Cu2S��CuS��
a��Cu2S��CuS���Ǻ�ɫ�ģ������ܺ��У���a���ϣ�
b��Cu��ŨH2SO4�ڼ��������£�ͭ����ԭ����Ũ����������������Ԫ���������У����Է�Ӧ�����ܷų�O2����b�����ϣ�
c��Ũ��������������ͭ�ǻ�ԭ����Cu���ϼ�������+1�ۻ�+2����S���ϼ��½���+6�۱仯Ϊ+4�ۣ�-2�۵��ж��ֿ��ܣ���c���ϣ�
�ʴ�Ϊ��a��c��
��5�����徻����ϴ�Ӻ�����أ�������Ҫ���������أ��������ʴ�Ϊ�����
��6������0.483g����CuO�����������պ�õ�Cu2O ������0.4347��������ֻ��0.420g�����Եó�һ�����и������պ�����С��0.420g�����ʣ�
����0.483g����Cu2S���������պõ�Cu2O����0.4347g��0.420g��
����0.483g����CuS���������պõ�Cu2O����0.36225g��0.420g��
���Ժ�ɫ���������ͭ�һ������CuS��
�ʴ�Ϊ��CuS��
���� ���⿼����Ũ�������ʵ�Ӧ�ã�Ϊ�߿��������ͣ���Ŀ�Ѷ��еȣ��漰ͭ��Ũ����ķ�Ӧ���������ʵ�̽����������������ʼ�����Ӧ�ã�������ؿ���ѧ���ķ���������������


 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�S2��S8�Ĺ������ﹲ6.4g������������ԭ����һ��Ϊ0.2NA | |
| B�� | ��1molFeCl3����������ˮ�У�����ɢϵ��������ĿС��NA | |
| C�� | 6.4gͭ����Ӧʱ��ͭʧȥ�ĵ�����Ϊ0.2NA | |
| D�� | ��״���£�44.8L NO��22.4L O2��Ϻ������з�������Ϊ2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Һ�е�SO42-������HCl�ữ��Ba��NO3��2��Һ���۲����ް�ɫ�������� | |
| B�� | ��ȥNO�л��е�����NO2�����������ͨ��ˮ�У������ſ������ռ�NO | |
| C�� | ��NaNO3������Ũ��������HNO3 | |
| D�� | ͨ��CO2�Գ�ȥNa2CO3��Һ�л��е�NaHCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ��ѧ���� | ʵ��Ӧ�� |
| A | Na��K���ǻ��ý��� | �ƼغϽ�����ԭ�ӷ�Ӧ�ѵ��ȼ� |
| B | H2O2��ʹ�����ʱ��� | ҽ���������˿����� |
| C | CH2=CH2����H2O�����ӳɷ�Ӧ | ��ϩ������ʵ�Ĵ���� |
| D | NH3���л�ԭ�� | ��ʳƷ�ӹ�������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ClO2�����������ˮ�У�ClO2��Ũ��Ӧ��0.10��0.80mg/L֮�䣮�õ��������ˮ��C1O2Ũ�ȵ�ʵ�鲽�����£�
��ClO2�����������ˮ�У�ClO2��Ũ��Ӧ��0.10��0.80mg/L֮�䣮�õ��������ˮ��C1O2Ũ�ȵ�ʵ�鲽�����£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 144.0gCuSO4 | B�� | 144.0gCuSO4•5H2O | ||
| C�� | 255.0gCuSO4•5H2O | D�� | 250.0gCuSO4•5H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� |  | C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com