
MgSO4��7H2O��һ����Ҫ�Ļ���ԭ�ϡ�ij�о���ѧϰС�������������þ��ʯ(��Ҫ�ɷ���MgCO3��������MnCO3��SiO2����)��ȡMgSO4��7H2O��ʵ�飬�������¡�
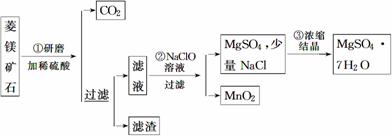
(1)�ڢٲ���ĥ��Ŀ����____________________��������Һ��Ҫ��__________________��__________________�����ʡ�
(2)�ڢڲ���Ӧ�����ӷ���ʽΪ_____________________________________________��
(3)�ڢ۲�Ũ���ᾧ��Ҫ����________��ϴ�ӡ�����Ȳ���ſɵõ�MgSO4��7H2O��ϴ�ӳ����Ļ���������______________________________________________________��
(4)���Ƶ�MgSO4��7H2O����Ϊ82.00 g�����MnO2����Ϊ1.74 g���Ҳ�õڢٲ���������Ϊ4.70 g�����Ը���������Һ�е��ܽ���ʧ���ɼ������þ��ʯ��MgCO3����������Ϊ____________________��
�𰸡�(1)ʹ�ܽ��ֲ��ӿ��ܽ����ʡ�MgSO4��MnSO4
(2)Mn2����ClO����H2O===MnO2����Cl����2H��
(3)���ˡ����������ڹ������У�������ˮ���պý�û��������ˮ�������ظ�2��3�� (4)80%
������(1)��ĥ���������ı������ʹ�ܽ������ܽⲢʹ�ܽ����ʼӿ졣��þ��ʯ��ϡ���ᷴӦ��MgCO3��MnCO3��ת��ΪMgSO4��MnSO4��(2)��������ͼ������NaClO��Һ��MnSO4ת��ΪMnO2�����ݵ�ʧ�����غ��Ԫ���غ㼴��д���ڢڲ���Ӧ�����ӷ���ʽ��Mn2����ClO����H2O===MnO2����Cl����2H����(3)�ڢ۲�Ũ���ᾧ��Ҫ�������ˡ�ϴ�ӡ�������ܵõ�MgSO4��7H2O���塣ϴ�ӳ���ʱҪע���ˮ���ܹ��࣬�Ҳ����ò��������裬��Ҫ�ظ�2��3�Ρ�(4)����������Ϣ�����MgCO3��MnCO3�������ֱ���
28��00 g��2.30 g������þ��ʯ��MgCO3����������Ϊ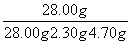 ��100%��80%��
��100%��80%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������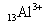 ��
�� ��Cl- ��
��Cl- ��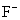 ��������������ͬ����
��������������ͬ����
�� A���٢ڢ� B���ڢۢ� C���٢ڢ� D���٢ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
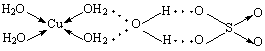
����ͭ�����ܽ����Һ������ɫ���μӰ�ˮֱ�����������յõ�����ɫ����Һ���ټ�����ˮ�Ҵ�����������ɫ���壬��ѧʽΪ��CuSO4•4NH3•H2O�����������ʵ������ش��������⣺
��1��д�������CuSO4•4NH3•H2O�е����壺 ����λ���ǣ� �����û�ѧʽ�����ֱ�ʾ����N��O��S����Ԫ�صĵ�һ�����ܴӴ�С��˳���� ��
��2����֪CuSO4•4NH3•H2O�Ľṹ��CuSO4•5H2O�������ƣ� CuSO4•5H2O����Ľṹ����ͼ��ʾ���뻭��CuSO4•4NH3•H2O�Ľṹʾ��ͼ��
 ��
��
��3����CuSO4•4NH3•H2O�����У�����ԭ����sp3�ӻ���ԭ���У� ��дԪ�ط��ţ���
�侧��ṹ�д��ڵĻ�ѧ���У� ��
A. ���»��� B. ������ C. ���ۼ� D. ��� E. ���Ӽ� F. ��λ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ú�����þ�۵�������ȡ�������������������в�����������ǡ��������� (����)
�ټ������ܽ⡡�ڼ��ռ���Һ�ܽ⡡�۹��ˡ���ͨ�����CO2����Al(OH)3�������ݼ�����������Al(OH)3���� ��������ռ���Һ
A���٢ޢݢ� B���ڢۢܢ�
C���ڢۢݢ� D���٢ۢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾAlCl3��Һ��NaOH��Һ��μӹ������������Ĺ�ϵ���ߣ������жϴ������ (����)
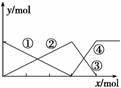
A�����߱�ʾAl3�������ʵ����ı仯
B��x��ʾAlCl3�����ʵ���
C�����߱�ʾAl(OH)3�����ʵ����ı仯
D�����߱�ʾ[Al(OH)4]�������ʵ����ı仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ѧ������̽������������Ϊ����Դ�Ŀ����ԣ��������ܳ�Ϊһ��ʯ�͵�ȡ�����������Ϊһ���ձ�ʹ�õ�������Դ���������ã��������������ص�����˵��������Ϊ�����Ǵ���� (����)
A�������ᣬ�������䡢���棬�Ұ�ȫ
B����ȼ��ʱ�ų�����������ȼ�պ����Ի�������Ⱦ���õ���Ч�Ŀ���
C���ڵ����ϣ�������Դ�ȽϷḻ
D���ִ���ұ���Ĺ�ҵ������Ϊ����Ϊ����Դ�춨����Ҫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
˫�ǻ���̼������ҽ���ϳ��õ�һ����������仯ѧʽ��NaAl(OH)2CO3�����ڸ����ʵ�˵����ȷ���� (����)
A������������������������
B����������Al(OH)3��Na2CO3�Ļ����
C��1 mol NaAl(OH)2CO3��������3 mol H��
D����ҩ�����ʺ���θ�����߷���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������ȷ���ǣ� �� ��
A����ԭ�ԣ�HI > HBr > HCl > HF
B�������۵㣺���ʯ > SiC > NaCl > Na > Mg > Al
C������: HClO4 > H2SO4 > H2SO3 > HClO
D�������ܣ� MgF2> NaCl > NaBr
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com