
�����ж���ȷ����(����)
A����ij��Һ�м���AgNO3��Һ���ɰ�ɫ����������ϡ����ʱ�������ܽ⣬��ȷ����Һ����Cl������
B����ij��Һ������ɫ��Ӧʱ������ɫΪ��ɫ�������Һ��һ����Na����������K��
C����������ʱ����ʹ����ʯ��ˮ����ǵ��������ɣ���ԭ��Һ��һ���д�����CO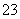 ����
����
D���ֱ���Mg2����Cu2����Fe2����Na������������Һ��ֻ��NaOH��Һ����һ���Լ������
 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ���� (�� ��)
A��Ԫ�صĵ���һ����������ԭ��Ԫ�صĻ������Ƶ�
B���ڻ�ѧ��Ӧ�У��õ���Խ������������������Ծ�Խǿ
C��������ֻ�ܵõ����ӱ���ԭ��������ֻ��ʧȥ���ӱ�����
D���������Ԫ�صĻ����ﲻһ�����к�ǿ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����жϣ���ȷ�Ĵ̡�������Ĵ���
(1)NH3��SO2��ˮ��Һ�����磬����NH3��SO2�����ڵ����(����)
(2)ǿ����ʱ�����Һһ����������ʱ�����Һ�ĵ�����ǿ(����)
(3)NaCl��Һ�ܵ��磬��NaCl��ҺΪ�����(����)
(4)Fe��Cu��Ag�ۻ��ܵ��磬��Fe��Cu��AgΪ�����(����)
(5)H2S����ˮ�ĵ��뷽��ʽΪ
H2SH����HS����HS��H����S2��(����)
(6)������������ˮ�ĵ��뷽��ʽΪ
NaHSO4Na����H����SO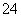 (����)
(����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ʵ�Ļ�ѧ������ȷ����(����)
A����NH3ͨ����з�̪��ˮ�У���Һ��죺NH3��H2ONH3·H2ONH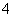 ��OH��
��OH��
B��ͭ��Ũ���Ṳ�Ȳ������壺Cu��H2SO4(Ũ)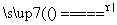 Cu2����SO
Cu2����SO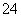 ��H2��
��H2��
C��CH3COONa��ˮ��Һ�ʼ��ԣ�CH3COO����H2O===CH3COOH��OH��
D����NaOH��Һ����Cl2��Cl2��2OH��===2Cl����H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ�������й㷺��Ӧ��ǰ��������ǰ��Ժ�ˮ����Ԥ������
(1)ͨ��������[K2SO4·Al2(SO4)3·24H2O]���������������Ƕȡ�����ˮ������ӷ���ʽ��____________________________________��
(2)����ͼ��ʾNaClO�ķ���װ�öԺ�ˮ�������������崦����
��װ������NaClת��ΪNaClO�����е����ӷ���ʽ__________________��__________________���ܵ����ӷ���ʽ______________��
�ں�ˮ�к���Ca2����Mg2����HCO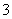 ���������ӣ�����������װ�õ������ײ���ˮ��������Ҫ�ɷ���Mg(OH)2��CaCO3������CaCO3�����ӷ���ʽ��____________________________��
���������ӣ�����������װ�õ������ײ���ˮ��������Ҫ�ɷ���Mg(OH)2��CaCO3������CaCO3�����ӷ���ʽ��____________________________��

����ÿ��5��10 min����һ�ε缫���ԣ�����Ч�ؽ�������ĽṸ���⡣
���õ缫��Ӧʽ����ϱ�Ҫ�����ֽ��н���____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ɫ��Һֻ���ܺ������¼������ӣ���Mg2������Al3������Fe2������H������HCO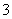 ����Cl������OH���������л����ص���NaOH��Һ���������������������������NaOH��Һ������Ĺ�ϵ��ͼ��ʾ���ɴ˿�ȷ��ԭ��Һ��һ�����е�������(����)
����Cl������OH���������л����ص���NaOH��Һ���������������������������NaOH��Һ������Ĺ�ϵ��ͼ��ʾ���ɴ˿�ȷ��ԭ��Һ��һ�����е�������(����)
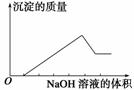
A���٢ڢ� B���٢ۢ�
C���ڢޢ� D���٢ڢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������������������������������
��֪���ȼ��a g��Ȳ����ʱ����1 mol������̼�����Һ̬ˮ�����ų�����b kJ������Ȳȼ�յ��Ȼ�ѧ����ʽ��ȷ����(����)
A��2C2H2(g)��5O2(g)===4CO2(g)��2H2O(l)����H����2b kJ·mol��1
B��C2H2(g)��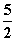 O2(g)===2CO2(g)��H2O(l)�� ��H��2b kJ·mol��1
O2(g)===2CO2(g)��H2O(l)�� ��H��2b kJ·mol��1
C��2C2H2(g)��5O2(g)===4CO2(g)��2H2O(l)����H����4b kJ·mol��1
D��2C2H2(g)��5O2(g)===4CO2(g)��2H2O(l)����H��b kJ·mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��CH3COOH��Һ�е���ϡ��ˮ����Һ�ĵ������������仯�������ǿ��(I)�氱ˮ�ļ������(V)�ı仯����(��ͼ)��(����)

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��20mL0.4mol/L�������Һ��50mL0.1mol/L����������Һ��ϣ�������Һ������Ũ�ȼ�Ĺ�ϵ��ȷ����( )
A��c(NO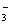 )>c(OH��)>c(NH
)>c(OH��)>c(NH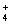 )>c(Ba2+) B��c(NO
)>c(Ba2+) B��c(NO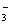 )>c(Ba2+)>c(NH
)>c(Ba2+)>c(NH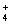 )>c(OH��)
)>c(OH��)
C��c(NO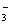 )=c(NH
)=c(NH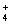 )+c(NH3��H2O)+c(NH3) D��2c(Ba2+)+c(H+)=c(NO
)+c(NH3��H2O)+c(NH3) D��2c(Ba2+)+c(H+)=c(NO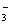 )+c(OH��)
)+c(OH��)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com