
PbO+C![]() Pb+CO(��Ҫ)��PbO+CO
Pb+CO(��Ҫ)��PbO+CO![]() Pb+
Pb+![]() ��
��
[Cu(NH3)2]Ac+CO+NH3![]() [Cu(NH3)3]Ac��CO +QkJ
[Cu(NH3)3]Ac��CO +QkJ
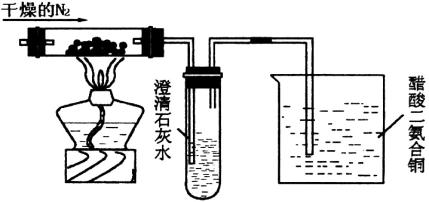
�ش������й����⣺
(1)ʢ����ʯ��ˮ���Թܿ�ʼһ��ʱ�����û��������ԭ����________��
(2)�ձ��д��������ͭ([Cu(NH3)2]��Ac)������Ϊ________��
(3)ʵ���Ĵ��������ͭ�����ʵ������ֿ�����������������������������________��
(4)���ÿ����������N2������________����������________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
ijѧ�����һ��ʵ��֤��PbO�к���������Ӧ����ʽ���£�
PbO+C![]() Pb+CO(��Ҫ)��PbO+CO
Pb+CO(��Ҫ)��PbO+CO![]() Pb+
Pb+![]() ��
��
[Cu(NH3)2]Ac+CO+NH3![]() [Cu(NH3)3]Ac��CO +QkJ
[Cu(NH3)3]Ac��CO +QkJ
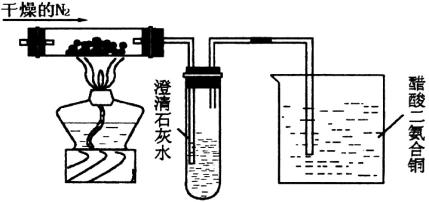
�ش������й����⣺
(1)ʢ����ʯ��ˮ���Թܿ�ʼһ��ʱ�����û��������ԭ����________��
(2)�ձ��д��������ͭ([Cu(NH3)2]��Ac)������Ϊ________��
(3)ʵ���Ĵ��������ͭ�����ʵ������ֿ�����������������������������________��
(4)���ÿ����������N2������________����������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�058

PbO+C![]() Pb+CO����Ҫ��
Pb+CO����Ҫ��
PbO+CO![]() Pb+CO2
Pb+CO2
[Cu(NH3)2]Ac+CO+NH3ƒ[Cu(NH3)3]Ac��CO+QkJ
�ش������й����⣺
��ʢ����ʯ��ˮ���Թܿ�ʼһ��ʱ�����û��������ԭ����________��
���ձ�����������ͭ([Cu(NH3)2]��Ac)������Ϊ________________��
�����ÿ����������N2������________��������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�058
��ͼ��ijѧ�����һ��ʵ��֤��PbO�к���������Ӧ����ʽ���£�

PbO+C![]() Pb+CO����Ҫ��
Pb+CO����Ҫ��
PbO+CO![]() Pb+CO2
Pb+CO2
[Cu(NH3)2]Ac+CO+NH3ƒ[Cu(NH3)3]Ac��CO+QkJ
�ش������й����⣺
��ʢ����ʯ��ˮ���Թܿ�ʼһ��ʱ�����û��������ԭ����________��
���ձ�����������ͭ([Cu(NH3)2]��Ac)������Ϊ________________��
�����ÿ����������N2������________��������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijѧ�����һ��ʵ��֤��PbO�к�����Ԫ�أ���Ӧʽ���£�
PbO+C ![]() Pb+CO����
Pb+CO����
PbO+CO![]() Pb+CO2
Pb+CO2
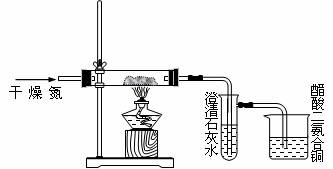
�ش��������⣺
��1����װ��ͼ���и����ԵĴ�����ָ����
��2��ʢ����ʯ��ˮ���Թܿ�ʼһ��ʱ�����û��������ָ�����ܵ�ԭ��
��3���ձ��д��������ͭ��������ʲô��
��4�����ÿ����������ĵ���������Ϊʲô��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com