
| A£® | ŗ¬ÓŠNH4+”¢Cl-”¢H+”¢OH-µÄČÜŅŗÖŠ£¬ĘäĄė×ÓÅضČŅ»¶ØŹĒ£ŗc£ØCl-£©£¾c£ØNH4+£©£¾c£ØH+£©£¼c£ØOH-£© | |
| B£® | pH=6µÄ“×ĖįÓė“×ĖįÄʵĻģŗĻČÜŅŗÖŠ£¬c£ØNa+£©£¾c£ØCH3COO-£© | |
| C£® | 0.1 mol/L µÄNa2SČÜŅŗÖŠ£¬c£ØOH£©=c£ØH+£©+c£ØHS-£©+2c£ØH2S£© | |
| D£® | pH=3µÄŅ»ŌŖĖįŗĶpH=11µÄŅ»ŌŖ¼īµČĢå»ż»ģŗĶŗóµÄČÜŅŗÖŠ£¬Ņ»¶ØŹĒc£ØOH-£©=c£ØH+£© |
·ÖĪö A”¢ŗ¬ÓŠNH4+”¢Cl-”¢H+”¢OH-µÄČÜŅŗĀś×ćµēŗÉŹŲŗć¼“æÉ£»
B”¢pH=6µÄ“×ĖįŗĶ“×ĖįÄʵĻģŗĻČÜŅŗĻŌĖįŠŌ£¬¼“c£ØH+£©£¾c£ØOH-£©£»
C”¢øł¾ŻÖŹ×ÓŹŲŗ楓·ÖĪö£»
D”¢pH=3µÄĖįŗĶpH=11µÄŅ»ŌŖ¼īµÄĒæČõĪ“ÖŖ£®
½ā“š ½ā£ŗA”¢ŗ¬ÓŠNH4+”¢Cl-”¢H+”¢OH-µÄČÜŅŗĀś×ćµēŗÉŹŲŗć¼“æÉ£¬¹ŹĄė×ÓÅضČæÉÄÜĪŖc£ØCl-£©£¾c£ØNH4+£©£¾c£ØH+£©£¾c£ØOH-£©£¬»¹æÉÄÜĪŖc£ØOH-£©£¾c£ØNH4+£©£¾c£ØH+£©£¾c£ØCl-£©£¬»ņŹĒc£ØCl-£©£¾c£ØH+£©£¾c£ØNH4+£©£¾c£ØOH-£©£¬¹ŹA“ķĪó£»
B”¢pH=6µÄ“×ĖįŗĶ“×ĖįÄʵĻģŗĻČÜŅŗĻŌĖįŠŌ£¬¼“c£ØH+£©£¾c£ØOH-£©£¬øł¾ŻµēŗÉŹŲŗćæÉÖŖ£¬c£ØNa+£©£¼c£ØCH3COO-£©¹ŹB“ķĪó£»
C”¢0.1 mol/L µÄNa2SČÜŅŗÖŠ£¬S2-½įŗĻĖ®µēĄė³öµÄĒāĄė×Ó¶ųĖ®½āĪŖHS-”¢H2S£¬µ¼ÖĀĖ®µēĄė³öµÄĒāĄė×ӵēęŌŚŠĪŹ½ÓŠČżÖÖ£ŗH+”¢HS-”¢H2S£¬¹Źøł¾ŻÖŹ×ÓŹŲŗćæÉÖŖ£ŗc£ØOH£©=c£ØH+£©+c£ØHS-£©+2c£ØH2S£©£¬¹ŹCÕżČ·£»
D”¢pH=3µÄĖįŗĶpH=11µÄŅ»ŌŖ¼īµÄĒæČõĪ“ÖŖ£¬¹ŹĮ½Õß»ģŗĻŗóµÄ£¬æÉÄÜĖį¹żĮ棬æÉÄܼī¹żĮ棬»¹æÉÄÜĒ”ŗĆĶźČ«·“Ó¦£¬¹Ź»ģŗĻŗóČÜŅŗµÄĖį¼īŠŌĪ“ÖŖ£¬¹ŹD“ķĪó£®
¹ŹŃ”C£®
µćĘĄ ±¾Ģāæ¼²éĮĖŃĪĄąĖ®½ā£¬øł¾ŻČÜŅŗĖį¼īŠŌŌŁ½įŗĻµēŗÉŹŲŗ楓·ÖĪö½ā“š£¬ÄѵćŹĒÖŹ×ÓŹŲŗćµÄŌĖÓĆŗĶĄķ½ā£®


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | FeCl3 | B£® | Na2S | C£® | £ØNH4£©2CO3 | D£® | Na2SO4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CH4 | B£® | CO | C£® | NO2 | D£® | Na2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ŹµŃé²Ł×÷ | ĻÖĻó | ½āŹĶ |
| A | ŌŚĆܱÕČŻĘ÷ÖŠ¼ÓČČCuOÖĘ1000”ę | ŗŚÉ«¹ĢĢå±ä³ÉŗģÉ«¹ĢĢå | CuOŹÜČČ·Ö½āµĆµ½µ„ÖŹCu |
| B | ½«SO2ĶØČėĘ·ŗģČÜŅŗÖŠ | ČÜŅŗĶŹÉ« | SO2¾ßÓŠĘư׊Ō |
| C | ½«Mg”¢AlÓėNaOHČÜŅŗ×é³ÉŌµē³Ų | Alµē¼«Čܽā | Al±ČMg½šŹō»ī¶ÆŠŌĒæ |
| D | ĻņijČÜŅŗÖŠ¼ÓČėŃĪĖįĖį»ÆµÄĀČ»Æ±µČÜŅŗ | ÓŠ°×É«³ĮµķÉś³É | øĆČÜŅŗÖŠŅ»¶Øŗ¬ÓŠSO42- |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ³£ĪĀ³£Ń¹ĻĀ£¬3.2 g O3Ėłŗ¬µē×ÓŹżĪŖ1.2 NA | |
| B£® | ±ź×¼×“æöĻĀ£¬2.24 L CCl4ÖŠŗ¬ÓŠµÄC-Cl¼üµÄŹżÄæĪŖ0.4 NA | |
| C£® | COÓėN2»„ĪŖµČµē×ÓĢ壬±ź×¼×“æöĻĀ11.2 L COÓė0.5 molN2Ėłŗ¬µē×ÓŹżĻąµČ | |
| D£® | ½«0.1 mol ĀČ»ÆĢśČÜÓŚ1 LĖ®ÖŠ£¬ĖłµĆČÜŅŗŗ¬ÓŠ0.1 NA Fe3+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
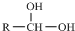 $\stackrel{-H_{2}O}{”ś}$
$\stackrel{-H_{2}O}{”ś}$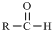 Ēėøł¾ŻČēĶ¼»Ų“š£ŗ
Ēėøł¾ŻČēĶ¼»Ų“š£ŗ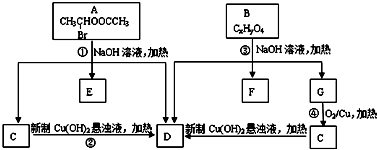
 £®
£® £»
£» £®
£®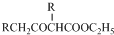 +C2H5OH£¬ĒėŅŌGĪŖĪØŅ»ÓŠ»śŹŌ¼ĮŗĻ³ÉŅŅõ£ŅŅĖįŅŅõ„£ØCH3COCH2COOC2H5£©£¬Éč¼ĘŗĻ³ÉĀ·ĻߣØĘäĖūŹŌ¼ĮČĪŃ”£©£®
+C2H5OH£¬ĒėŅŌGĪŖĪØŅ»ÓŠ»śŹŌ¼ĮŗĻ³ÉŅŅõ£ŅŅĖįŅŅõ„£ØCH3COCH2COOC2H5£©£¬Éč¼ĘŗĻ³ÉĀ·ĻߣØĘäĖūŹŌ¼ĮČĪŃ”£©£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com