
19.2g Cu�������50 mL 18 mol��L��1Ũ���������ȫ��Ӧ��
��Ӧ����ʽ���£� Cu +
2H2SO4��Ũ��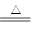 CuSO4 +
SO2��+ 2H2O ��
CuSO4 +
SO2��+ 2H2O ��
��1���õ�SO2������������״���£��� L��
��2������Ӧ����Һ�м���ˮ����Һ�����Ϊ500mL��ȡ��50mL����ô50mL��Һ��CuSO4 �����ʵ���Ũ��Ϊ mol��L-1��
��3������Ӧ����Һ�м���������BaCl2�������������ᱵ����Ϊ g��
��1��6. 72 ��2�� 0.6 ��3�� 139.8
��������
���������19.2gCuΪ0.3mol��������Ũ��������0.3molSO2��ͬʱ����0.3molCuSO4������Һϡ����500mL�����ʵ���Ũ��Ϊ0.6mol/L����Ӧ����Һ��SO42-���ʵ���Ϊ(0.05��18-0.3)mol=0.6mol,��Ӧ�����Һ�м��������Ȼ�����Һ�������ᱵ0.6mol��
���㣺��ѧ����
���������⣨3��Ӧ��Ԫ���غ��˼�롣��Ӧǰ�������ʵ���Ϊ0.9mol������SO2��SΪ0.3mol�����Բ�����Һ��SO42-Ϊ0.6mol��


 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ�鲽�� | ʵ������ |
| ��1��ȡ��������Һ���ⶨ��ҺpH | pH=0 |
| ��2��ȡ��������Һ����Ũ������CuƬ��Ũ H2SO4������ | ����ɫ���������������������ɺ���ɫ |
| ��3��ȡ��������Һ��������BaCl2��Һ | �а�ɫ���� |
| ��4��ȡ��3�����ϲ���Һ��������AgNO3��Һ | �а�ɫ�������Ҳ�����ϡHNO3 |
| ��5��ȡ��������Һ����NaOH��Һ | �а�ɫ������NaOH����ʱ���������ܽ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com