
(8��)����β����Ҫ����![]() ��
��![]() ��
��![]() ��
��![]() �����͡�����(��
�����͡�����(��![]() ��
��![]() Ԫ�����)�����ʣ�����β��Խ��Խ��Ϊ���п�����Ⱦ����Ҫ��Դ���������������������ķ���֮һ������������������װһ������ת������(�ò����ٺϽ�������)�������ص���ʹ
Ԫ�����)�����ʣ�����β��Խ��Խ��Ϊ���п�����Ⱦ����Ҫ��Դ���������������������ķ���֮һ������������������װһ������ת������(�ò����ٺϽ�������)�������ص���ʹ![]() ��
��![]() ��Ӧ�����ɿɲ��������̬����ѭ���Ķ��������壬����ʹ���͡����͵����ʳ��ȼ�ռ�
��Ӧ�����ɿɲ��������̬����ѭ���Ķ��������壬����ʹ���͡����͵����ʳ��ȼ�ռ�![]() ��ת����
��ת����
(1)����β����![]() ����Դ�� ��
����Դ�� ��
A�����͡����͵�ȼ�ղ���
B���ǿ����е�![]() �����͡����͵ķ�Ӧ����
�����͡����͵ķ�Ӧ����
C���ǿ����е�![]() ��
��![]() �����������ڵĸ��»����µķ�Ӧ����
�����������ڵĸ��»����µķ�Ӧ����
D������������β�������˿�����![]() ��
��![]() �Ļ��Ϸ�Ӧ
�Ļ��Ϸ�Ӧ
(2)ָ��![]() ��
��![]() ��Ӧ�е��������� ������������ ��
��Ӧ�е��������� ������������ ��
(3)����ת��������ȱ������һ���̶�������˿�������ȣ���ԭ����
(4)���ƻ����ٳ�������β����Ⱦ�ķ��������� ��
A����������Դ B��ʹ�õ綯�� C����ֹ������ʻ D��ʹ���Ҵ�����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| �� |
 �������������������������������ռ�����
�������������������������������ռ�����| ���� |
| ���� |
| ���� |
| ���� |
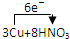 =3Cu��NO3��2+2NO��+4H2O
=3Cu��NO3��2+2NO��+4H2O =3Cu��NO3��2+2NO��+4H2O
=3Cu��NO3��2+2NO��+4H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��)![]() �����Ѿ���Ϊ��Ҫ�Ľ�ͨ���ߣ������ŷŵ�β���ǿ�������Ҫ��Ⱦ��֮һ����֪����β���е���Ҫ��Ⱦ���У�CmHn(��)��SO2��NOX��CO��C�ȣ���ش������й����⡣
�����Ѿ���Ϊ��Ҫ�Ľ�ͨ���ߣ������ŷŵ�β���ǿ�������Ҫ��Ⱦ��֮һ����֪����β���е���Ҫ��Ⱦ���У�CmHn(��)��SO2��NOX��CO��C�ȣ���ش������й����⡣
(1)����CmHn��ʾ���͵���Ҫ��ɣ�CmHn�ڿ�������ȫȼ�յĻ�ѧ����ʽΪ ������ȼ�ղ�������Ϊ�����ṩ�˶�������һ������������ת������ ��ת��Ϊ �ܣ�����ת��Ϊ��е�ܣ�
(2)ͨ������ȼ�͵ľ����ӹ��������ɼ�������β���е� (�ѧʽ������ղ��÷�)�ŷţ�
(3)Ŀǰ����β������ô�ת���ķ�������������д���ڴ���������NOX��CO��Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ӱ�ʡ�����߿�����������ԣ�һ�������ۣ���ѧ���� ���ͣ�ʵ����
I������ʵ��������Լ����淽����ʵ�����¹ʴ�����һ����ȷ���� ������ţ���
A��ʵ�����У�Ũ���ᱣ���ڴ���������ɫϸ���Լ����У�
B���Ʊ�������������ʱ��Ӧ��20mL��ˮ����εμ�1~2mL���͵�FeCl3��Һ�����������ȵ�Һ������ĺ��ɫΪֹ��
C����ʯ�͵ķ���ʵ���У��¶ȼ������Һ���У�
D��������Ũ��Һմ��Ƥ���ϣ�Ҫ�����ô���ˮ��ϴ��Ȼ��Ϳ��������Һ��
E����ʽ�ζ�����ȡ20.00mL�������������Һ��
F���ڽ����к��Ȳⶨʱ��Ϊ��֤ʵ���ȷ�ԣ����ǿ��Բ�ȡ���¾����ʩ��ʹ������ĭ�����ȱ��µ����á�ʹ��ͭ�ʽ�������н��衢ȡ�õļ���Һ�Թ�����������������ʵ��ȡƽ��ֵ��
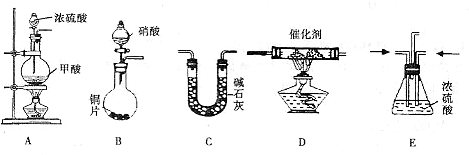
II������β������Ҫ�ɷ�ΪCO2��CO��NOX��NO��NO2���Ļ�������NO���������ռ95%���ϣ��ȡ���������֮һ���������������ϼ�װ����ת�����������ô���ʹCO��NOX������Ӧ��ת��ΪCO2��N2��ijС����ʵ����������ͼ��ʾװ��ģ�������β����CO��NOX�ķ�Ӧ��������Ӧ�����������ɡ�����֪ ��
��

�Իش��������⣺
(1)����������˳��Ϊ
(2)Eװ�õ�������___________________��___________________________________________��
(3)д��D�е�NOX��CO��Ӧ�Ļ�ѧ����ʽ______________________________________��
(4)��B���������ɵ�NOXΪNO��д���÷�Ӧ�Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ_________________________________________________________��
(5)��ͨ���NOXΪNO��Cװ������8.8g���ռ����������ڱ�״����Ϊ4.48L������Է�������Ϊ28.4�������ռ�����������NO�����ʵ���Ϊ___________________��
(6)ѡ���Ч������������β��ת��Ϊ�����壬�㳹���������β���Ի�����Ӱ�죬����˵���Ƿ���ȷ���������ɣ�___________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 CO��+H2O��
CO��+H2O��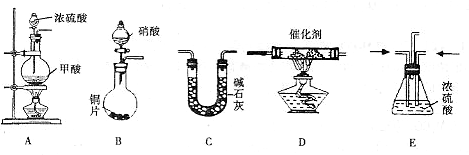
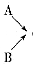 �������ռ�����
�������ռ������鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com