
·ÖĪö £Ø1£©¼īŹ½Ģ¼ĖįÄĘĀĮ[NaaAlb£ØOH£©c£ØCO3£©d]ÖŠ£¬»ÆŗĻ¼Ū“śŹżŗĶĪŖ0£»
£Ø2£©¼īŹ½Ģ¼ĖįÄĘĀĮ×÷ĪŖ×čČ¼¼ĮµÄæÉÄÜŌŅņ£ŗ¢ŁŌŚ·Ö½ā¹ż³ĢÖŠ“óĮæĪüČČ£»¢Ś±¾Éķ¼°²śĪļĪŽ¶¾ĒŅ²»æÉČ¼£»¢ŪĶ¬²śÉś²»Ö§³ÖČ¼ÉյĶžŃõ»ÆĢ¼ŗĶĖ®£»
£Ø3£©pH¹żøߣ¬¼īŠŌĒæ²»Éś³ÉĒāŃõ»ÆĀĮ£¬¶ų²śÉśĘ«ĀĮĖįŃĪ£»
£Ø4£©ŅņĪŖ£ŗn£ØCO2£©=$\frac{0.448L}{22.4L/mol}$£¬n£ØCO2£©=0.02mol£¬ĖłŅŌn£ØH2O£©=$\frac{2.880g”Į£Ø1-56.9%£©-0.02mol”Į44g/mol}{18g/mol}$£¬¶ų²āµĆČÜŅŗÖŠŗ¬ÓŠ0.02molAl3+£¬ĖłŅŌb£ŗc£ŗd=1£ŗ2£ŗ1£¬øł¾ŻµēŗÉŹŲŗća+0.02”Į3=0.02”Į2+0.02”Į2£¬ĖłŅŌa=0.02mol£¬ĖłŅŌa£ŗb£ŗc£ŗd=1£ŗ1£ŗ2£ŗ1£¬ÓÉ“Ė·ÖĪö½ā“š£®
½ā“š ½ā£ŗ£Ø1£©¼īŹ½Ģ¼ĖįÄĘĀĮ[NaaAlb£ØOH£©c£ØCO3£©d]ÖŠ£¬»ÆŗĻ¼Ū“śŹżŗĶĪŖ0£¬ĖłŅŌa+3b-c-2d=0£¬Ōņa+3b=c+2d£¬¹Ź“š°øĪŖ£ŗa+3b=c+2d£»
£Ø2£©¼īŹ½Ģ¼ĖįÄĘĀĮ×÷ĪŖ×čČ¼¼ĮµÄæÉÄÜŌŅņ£ŗ¢ŁŌŚ·Ö½ā¹ż³ĢÖŠ“óĮæĪüČČ£»¢Ś±¾Éķ¼°²śĪļĪŽ¶¾ĒŅ²»æÉČ¼£»¢ŪĶ¬²śÉś²»Ö§³ÖČ¼ÉյĶžŃõ»ÆĢ¼ŗĶĖ®£¬
¹Ź“š°øĪŖ£ŗ²śÉś×čČ¼ŠŌĘųĢåCO2”¢H2O£»
£Ø3£©pH¹żøߣ¬¼īŠŌĒæ²»Éś³ÉĒāŃõ»ÆĀĮ£¬¶ų²śÉśĘ«ĀĮĖįŃĪ£¬ĖłŅŌpH¹żøߣ¬Ōņ¶Ō²śĘ·µÄÓ°ĻģŹĒ»įŹ¹¼īŹ½Ģ¼ĖįÄĘĀĮ×Ŗ»ÆĪŖNaAlO2£¬
¹Ź“š°øĪŖ£ŗpH¹żøß»įŹ¹¼īŹ½Ģ¼ĖįÄĘĀĮ×Ŗ»ÆĪŖNaAlO2£»
£Ø4£©ŅņĪŖ£ŗn£ØCO2£©=$\frac{0.448L}{22.4L/mol}$£¬n£ØCO2£©=0.02mol£¬ĖłŅŌn£ØH2O£©=$\frac{2.880g”Į£Ø1-56.9%£©-0.02mol”Į44g/mol}{18g/mol}$£¬¶ų²āµĆČÜŅŗÖŠŗ¬ÓŠ0.02molAl3+£¬ĖłŅŌb£ŗc£ŗd=1£ŗ2£ŗ1£¬øł¾ŻµēŗÉŹŲŗća+0.02”Į3=0.02”Į2+0.02”Į2£¬ĖłŅŌa=0.02mol£¬ĖłŅŌa£ŗb£ŗc£ŗd=1£ŗ1£ŗ2£ŗ1£¬ĖłŅŌ£¬¼īŹ½Ģ¼ĖįĀĮµÄ»Æѧ×é³ÉĪŖNaAl£ØOH£©2CO3£¬
“š£ŗŅņĪŖ£ŗn£ØCO2£©=$\frac{0.448L}{22.4L/mol}$=0.02 mol””£Ø1·Ö£© n£ØCO2£©=0.02 mol
ĖłŅŌ£ŗn£ØH2O£©=$\frac{2.880g”Į£Ø1-56.9%£©-0.02mol”Į44g/mol}{18g/mol}$=0.02 mol
b£ŗc£ŗd=1£ŗ2£ŗ1£¬øł¾ŻµēŗÉŹŲŗć£¬a£ŗb£ŗc£ŗd=1£ŗ1£ŗ2£ŗ1£¬
ĖłŅŌ£¬¼īŹ½Ģ¼ĖįĀĮµÄ»Æѧ×é³ÉĪŖNaAl£ØOH£©2CO3 £»
µćĘĄ ±¾Ģāæ¼²é»Æѧ·½³ĢŹ½ÓŠ¹Ų¼ĘĖć£¬ĪŖøßĘµæ¼µć£¬Ć÷Č·ø÷øöĪļĄķĮæÖ®¼ä¹ŲĻµŹĒ½ā±¾Ģā¹Ų¼ü£¬×¢ŅāŌ×ÓŹŲŗćµÄĮé»īŌĖÓĆ£¬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĒāŃõ»ÆÄĘŗĶĘĻĢŃĢĒ·Ö±šČܽāŌŚĖ®ÖŠ | B£® | øɱłŗĶĀČ»Æļ§·Ö±šŹÜČȱäĪŖĘųĢå | ||
| C£® | Ź³ŃĪŗĶ±ł·Ö±šŹÜČČČŪ»Æ | D£® | ŅŗäåŗĶ¾Ę¾«·Ö±š»Ó·¢ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŗ¬ÓŠ¹²¼Ū¼üµÄ»ÆŗĻĪļŅ»¶ØŹĒ¹²¼Ū»ÆŗĻĪļ | |
| B£® | ŌŚ¹²¼Ū»ÆŗĻĪļÖŠŅ»¶Øŗ¬ÓŠ¹²¼Ū¼ü | |
| C£® | ŗ¬ÓŠĄė×Ó¼üµÄ»ÆŗĻĪļŅ»¶ØŹĒĄė×Ó»ÆŗĻĪļ | |
| D£® | ·Ē¼«ŠŌ¼üŅ²æÉ“ęŌŚÓŚĄė×Ó»ÆŗĻĪļÖŠ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
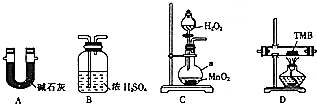 ÓÉĢ¼”¢Ēā”¢µŖČżÖÖŌŖĖŲ×é³ÉµÄTMBŹĒŅ»ÖÖŠĀŠĶÖøĪĘ¼ģ²āµÄÉ«ŌŹŌ¼Į£¬ĘäĻą¶Ō·Ö×ÓÖŹĮæĪŖ240£®Ä³ŃŠ¾æŠŌѧĻ°Š”×éµÄĶ¬Ń§ÓūĄūÓĆĻĀĮŠ×°ÖĆ²ā¶ØTMBµÄ·Ö×ÓŹ½£®ŹµŃéŌĄķ£ŗŌŚ×ćĮæŃõĘųĮ÷ÖŠ½«4.80gTMBѳʷŃõ»Æ£ØµŖŌŖĖŲ×Ŗ»ÆĪŖN2£©£¬ŌŁĄūÓĆĪüŹÕ¼Į·Ö±šĪüŹÕĖ®ÕōĘųŗĶCO2£®Ēė“ÓĶ¼ÖŠŃ”ŌńŹŹµ±µÄ×°ÖĆ£Ø²æ·Ö×°ÖĆæÉŅŌÖŲø“£©½ųŠŠŹµŃ飮
ÓÉĢ¼”¢Ēā”¢µŖČżÖÖŌŖĖŲ×é³ÉµÄTMBŹĒŅ»ÖÖŠĀŠĶÖøĪĘ¼ģ²āµÄÉ«ŌŹŌ¼Į£¬ĘäĻą¶Ō·Ö×ÓÖŹĮæĪŖ240£®Ä³ŃŠ¾æŠŌѧĻ°Š”×éµÄĶ¬Ń§ÓūĄūÓĆĻĀĮŠ×°ÖĆ²ā¶ØTMBµÄ·Ö×ÓŹ½£®ŹµŃéŌĄķ£ŗŌŚ×ćĮæŃõĘųĮ÷ÖŠ½«4.80gTMBѳʷŃõ»Æ£ØµŖŌŖĖŲ×Ŗ»ÆĪŖN2£©£¬ŌŁĄūÓĆĪüŹÕ¼Į·Ö±šĪüŹÕĖ®ÕōĘųŗĶCO2£®Ēė“ÓĶ¼ÖŠŃ”ŌńŹŹµ±µÄ×°ÖĆ£Ø²æ·Ö×°ÖĆæÉŅŌÖŲø“£©½ųŠŠŹµŃ飮²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ÄæµÄ | ²Ł×÷ |
| A | ÅäÖĘ100mL1.0mol•L-1CuSO4 ČÜŅŗ | ½«25.0gCuSO4•5H2OČÜÓŚÕōĮóĖ®Åä³É100mLČÜŅŗ |
| B | ³żČ„KNO3¹ĢĢåÖŠÉŁĮæNaCl | ½«»ģŗĻĪļÖĘ³ÉČȵı„ŗĶČÜŅŗ£¬ĄäČ“½į¾§£¬¹żĀĖ |
| C | ¼ģŃéČÜŅŗŹĒ·ńŗ¬ÓŠSO42- | ȔɣĮæ“ż²āŅŗÓŚŹŌ¹ÜÖŠ£¬¼ÓČėĻõĖįĖį»ÆµÄBa£ØNO3£©2ČÜŅŗ |
| D | ¼ģŃéČÜŅŗÖŠŹĒ·ńŗ¬ÓŠNH4+ | ȔɣĮæČÜŅŗÓŚŹŌ¹ÜÖŠ£¬¼ÓČėNaOHŗ󣬼ÓČČ£¬ŌŚŹŌ¹ÜæŚ·ÅÖĆŅ»Ę¬ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
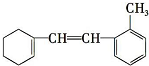
| A£® | øĆĪļÖŹ±½»·ÉĻµÄČżĀČ“śĪļÓŠ 4 ÖÖ | |
| B£® | 1 mol øĆĪļÖŹŗĶ H2¼Ó³É×ī¶ąŠčŅŖH2µÄĪļÖŹµÄĮæĪŖ 2 mol | |
| C£® | ÄÜŹ¹äåĖ®ĶŹÉ«£¬1 mol øĆĪļÖŹŗĶäåĖ®»ģŗĻ£¬×ī¶ąĻūŗÄ Br2µÄĪļÖŹµÄĮæĪŖ 5 mol | |
| D£® | øĆĪļÖŹÄŃČÜÓŚĖ®£¬ÄÜŹ¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ«£¬ĒŅ·¢ÉśµÄŹĒ¼Ó³É·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĄūÓĆĢ«ŃōÄÜ”¢³±Ļ«ÄÜ”¢·ēĮ¦·¢µē£¬æÉŅŌ»ńČ”Ēå½ąÄÜŌ“ | |
| B£® | Ź³ÓĆÓĶŗĶĘūÓĶ¶¼ŹōÓŚõ„Ąą£¬¶¼ÄÜÓĆĄ“¹¤ŅµÉĻÖĘ·ŹŌķ | |
| C£® | ĄūÓĆæɽµ½āµÄ”°ÓńĆ×ĖÜĮĻ”±Éś²śŅ»“ĪŠŌ·¹ŗŠ£¬æÉ·ĄÖ¹°×É«ĪŪČ¾ | |
| D£® | µŲ¹µÓĶČō±»»ŲŹÕÖŲŠĀĮ÷Čė²Ķץ£¬¶ŌČĖĢåÉĖŗ¦¼«“ó£¬Ó¦³«µ¼¼Ó¹¤“¦ĄķÉś³ÉÉśĪļ²ńÓĶ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com