
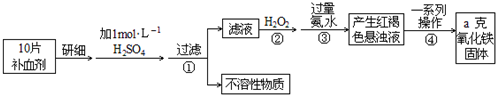
£Ø10·Ö£©FeŹĒČĖĢå²»æÉȱɣµÄĪ¢ĮæŌŖĖŲ£¬ÉćČėŗ¬Ģś»ÆŗĻĪļæɲ¹³äĢś”£ĮņĖįŃĒĢś¾§Ģå£ØFeSO4”¤7H2O£©ŌŚŅ½Ņ©ÉĻ×÷²¹ŃŖ¼Į”£Ä³æĪĶāŠ”×é²ā¶ØøĆ²¹ŃŖ¼ĮÖŠĢśŌŖĖŲµÄŗ¬Į攣ŹµŃé²½ÖčČēĻĀ£ŗ
£Ø1£©²½Öč¢Ś¼ÓČė¹żĮæH2O2µÄÄæµÄ£ŗ £»
£Ø2£©²½Öč¢ŪÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ £»
£Ø3£©²½Öč¢ÜÖŠŅ»ĻµĮŠ“¦ĄķµÄ²Ł×÷²½Öč£ŗ ”¢Ļ“µÓ”¢ ”¢ĄäČ“”¢³ĘĮ攣
£Ø4£©ŹµŃéÖŠÓĆÅØĮņĖįÅäÖĘ1 mol/£ĢµÄĻ”ĮņĖį£¬ÅäÖĘŹ±ÓƵ½µÄ¶ØĮæ²£Į§ŅĒĘ÷ÓŠ ”¢ ”£
£Ø5£©ČōŹµŃéĪŽĖšŗÄ£¬ŌņĆæʬ²¹ŃŖ¼Įŗ¬ĢśŌŖĖŲµÄÖŹĮæ g£ØÓĆŗ¬aµÄ“śŹżŹ½±ķŹ¾£©”£
£Ø1£©½«Fe2+Č«²æŃõ»ÆĪŖFe3+
£Ø2£©Fe3++3NH3”¤H2O=Fe(OH)3”ż+3NH4+
£Ø3£©¹żĀĖ ×ĘÉÕ
(4) ČŻĮæĘæ ĮæĶ²
£Ø5£©0.07a
½āĪö:£Ø1£©ĀĖŅŗÖŠŗ¬ÓŠŃĒĢśĄė×Ó£¬¼ÓČėĖ«ŃõĖ®æÉŅŌ½«ĘäŃõ»ÆÉś³ÉĢśĄė×Ó£¬ŅŌĄūÓŚ³Įµķ”£
£Ø2£©ĢśŃĪŗĶ¼ī·“Ó¦¾ĶæÉŅŌÉś³ÉĒāŃõ»ÆĢś³Įµķ”£
£Ø3£©ĒāŃõ»ÆĢś²»ČÜÓŚĖ®£¬æÉŅŌĶعż¹żĀĖµĆµ½¹ĢĢ壬Ėł¹żĀĖ³öĄ“µÄĒāŃõ»ÆĢśŠčŅŖĶعżĻ“µÓ£¬Č»ŗó×ĘÉÕ¼“×Ŗ»ÆĪŖŃõ»ÆĢś”£
£Ø4£©ÅØĮņĖįÅäÖĆĻ”ĮņĖįŠčŅŖĮæĶ²ŗĶČŻĮæĘæÕāĮ½ÖÖ¶ØĮæ²£Į§ŅĒĘ÷”£
£Ø5£©agŃõ»ÆĢśÖŠŗ¬ÓŠĢśŌŖĖŲµÄÖŹĮæŹĒ![]() £¬ĖłŅŌĆæʬ֊ŗ¬ÓŠµÄĢ¼ŌŖĖŲµÄÖŹĮæŹĒ
£¬ĖłŅŌĆæʬ֊ŗ¬ÓŠµÄĢ¼ŌŖĖŲµÄÖŹĮæŹĒ![]() ”£
ӣ


 ÅąÓÅæŚĖćĢāæØĻµĮŠ“š°ø
ÅąÓÅæŚĖćĢāæØĻµĮŠ“š°ø æŖŠÄæŚĖćĢāæØĻµĮŠ“š°ø
æŖŠÄæŚĖćĢāæØĻµĮŠ“š°ø æŚĖćĢāæØŗÓ±±ÉŁÄź¶łĶƳö°ęÉēĻµĮŠ“š°ø
æŚĖćĢāæØŗÓ±±ÉŁÄź¶łĶƳö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
FeŹĒČĖĢå²»æÉȱɣµÄĪ¢ĮæŌŖĖŲ£¬ÉćČėŗ¬Ģś»ÆŗĻĪļæɲ¹³äĢś”£
¢Å Õż³£ČĖĆæĢģÓ¦²¹³ä14mg×óÓŅµÄĢś”£ĘäÖŠ¾ų“ó²æ·ÖĄ“×ŌÓŚŹ³Īļ”£Čē¹ūČ«²æĶعż·žÓĆŗ¬FeSO4”¤7H2OµÄʬ¼ĮĄ“²¹³äĢś£¬ŌņÕż³£ČĖĆæĢģ·žŠčÓĆŗ¬ mg FeSO4”¤7H2OµÄʬ¼Į”£
¢Ę ”°ĖŁĮ¦·Ę”±ŹĒŹŠ³”ÉĻŅ»ÖÖ³£¼ūµÄ²¹ĢśŅ©Īļ£¬ĘäÖŠFe2£«µÄŗ¬ĮæĪŖ35.0%”£øĆŅ©Ę·ÖŠFe2£«»į»ŗĀżŃõ»Æ”£¹ś¼Ņ¹ę¶ØøĆŅ©ĪļÖŠFe2£«µÄŃõ»ÆĀŹ³¬¹ż10£„¼“²»ÄÜŌŁ·žÓĆ”£ĪŖ¼ģŃéijŅ©µź³öŹŪµÄ”°ĖŁĮ¦·Ę”±ŹĒ·ńŹ§Š§£¬Č”10.00gøĆŅ©Ę·Č«²æČÜÓŚĻ”ĮņĖį£¬ÅäÖĘ³É1000mLČÜŅŗ”£Č”ĘäÖŠ20.00mL£¬ÓĆ0.01000 mol/L KMnO4ČÜŅŗµĪ¶Ø£¬ÓĆČ„KMnO4ČÜŅŗ24.00mL”£Ķعż¼ĘĖćĖµĆ÷øĆŅ©ĪļŹĒ·ńÄÜ·žÓĆ£æ£ØMnO4£ŌŚĖįŠŌĢõ¼žĻĀµÄ»¹Ō²śĪļĪŖMn2£«£¬Ņ©Ę·ÖŠ³żFeŌŖĖŲĶāĘäĖū³É·Ö²»ÓėKMnO4·“Ó¦£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äź¹ć¶«Ź”Įś“ØŅ»ÖŠøßČż8ŌĀŌĀæ¼£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗĢīæÕĢā
£Ø15·Ö£©Ģś¼°Ęä»ÆŗĻĪļŌŚÉś»ī”¢Éś²śÖŠÓŠ¹ć·ŗÓ¦ÓĆ”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©FeŹĒČĖĢå²»æÉȱɣµÄĪ¢ĮæŌŖĖŲ£¬ÉćČėŗ¬Ģś»ÆŗĻĪļæɲ¹³äĢś”£Õż³£ČĖĆæĢģÓ¦²¹³ä14mg×óÓŅµÄĢś”£ĘäÖŠ¾ų“ó²æ·ÖĄ“×ŌÓŚŹ³Īļ”£Čē¹ūČ«²æĶعż·žÓĆŗ¬FeSO4”¤7H2OµÄʬ¼ĮĄ“²¹³äĢś£¬ŌņÕż³£ČĖĆæĢģ·žŠčÓĆŗ¬ mg FeSO4”¤7H2OµÄʬ¼Į”£
£Ø2£©Ä³Ķ¬Ń§ĪŖĮĖ¼ģŃé¼ŅÖŠµÄŅ»Ęæ²¹ĢśŅ©£Ø³É·ÖĪŖFeSO4£©ŹĒ·ń±äÖŹ£¬²éŌÄĮĖÓŠ¹Ų׏ĮĻ£¬µĆÖŖFe2+Äܱ»ĖįŠŌøßĆĢĖį¼ŲČÜŅŗŃõ»Æ¶ųŹ¹øßĆĢĖį¼ŲČÜŅŗĶŹÉ«£¬²¢½įŗĻŅŃѧµÄÖŖŹ¶Éč¼ĘĮĖČēĻĀŹµŃé£ŗ½«Ņ©Ę¬³żČ„ĢĒŅĀŃŠĻøŗó£¬Čܽā¹żĀĖ£¬Č”ĀĖŅŗ·Ö±š¼ÓČėĮ½Ö§ŹŌ¹ÜÖŠ£¬ŌŚŅ»Ö§ŹŌ¹ÜÖŠµĪČėĖįŠŌøßĆĢĖį¼ŲČÜŅŗ£¬ŌŚĮķŅ»Ö§ŹŌ¹ÜÖŠµĪČėKSCNČÜŅŗ”£øĆĶ¬Ń§¹Ū²ģµ½µÄĻÖĻóŹĒ£ŗµĪČėĖįŠŌøßĆĢĖį¼ŲČÜŅŗŗóĶŹÉ«£¬µĪČėKSCNČÜŅŗŗó²»±äŗģ£¬¾Ż“ĖµĆ³öµÄ½įĀŪŹĒ ”£
£Ø3£©»ĘĢśæó£ØFeS2£©ŹĒÉś²śĮņĖįŗĶŅ±Į¶øÖĢśµÄÖŲŅŖŌĮĻ”£ĘäÖŠŅ»øö·“Ó¦ĪŖ
3FeS2£«8O2 6SO2£«Fe3O4£¬ČōÓŠ3 mol FeS2²Ī¼Ó·“Ó¦£¬Ōņ×ŖŅĘ molµē×Ó”£
6SO2£«Fe3O4£¬ČōÓŠ3 mol FeS2²Ī¼Ó·“Ó¦£¬Ōņ×ŖŅĘ molµē×Ó”£
£Ø4£©ĀČ»ÆĢśČÜŅŗ³£ÓĆ×÷Ó”Ė¢µēĀ·Ķ°åøÆŹ“¼Į£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ £»
£Ø5£©ÓėĆ÷·ÆĻąĖĘ£¬ĮņĖįĢśŅ²æÉÓĆ×÷¾»Ė®¼Į£¬ŌŚŹ¹ÓĆŹ±·¢ĻÖĮņĖįĢś²¢²»ÄÜŹ¹ĖįŠŌ·ĻĖ®ÖŠµÄŠüø”Īļ³Į½µ³żČ„£¬ĘäŌŅņŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗŌĄŃōŹŠ2010½ģøßČżµŚĖÄ“ĪÖŹ¼ģæ¼ŹŌ£Ø»Æѧ£©ŹŌĢā ĢāŠĶ£ŗ¼ĘĖćĢā
(8·Ö) FeŹĒČĖĢå²»æÉȱɣµÄĪ¢ĮæŌŖĖŲ£¬ÉćČėŗ¬Ģś»ÆŗĻĪļæɲ¹³äĢś”£
¢Å Õż³£ČĖĆæĢģÓ¦²¹³ä14mg×óÓŅµÄĢś”£ĘäÖŠ¾ų“ó²æ·ÖĄ“×ŌÓŚŹ³Īļ”£Čē¹ūČ«²æĶعż·žÓĆŗ¬FeSO4”¤7H2OµÄʬ¼ĮĄ“²¹³äĢś£¬ŌņÕż³£ČĖĆæĢģ·žŠčÓĆŗ¬ mg FeSO4”¤7H2OµÄʬ¼Į”£
¢Ę ”°ĖŁĮ¦·Ę”±ŹĒŹŠ³”ÉĻŅ»ÖÖ³£¼ūµÄ²¹ĢśŅ©Īļ£¬ĘäÖŠFe2£«µÄŗ¬ĮæĪŖ35.0%”£øĆŅ©Ę·ÖŠFe2£«»į»ŗĀżŃõ»Æ”£¹ś¼Ņ¹ę¶ØøĆŅ©ĪļÖŠFe2£«µÄŃõ»ÆĀŹ³¬¹ż10£„¼“²»ÄÜŌŁ·žÓĆ”£ĪŖ¼ģŃéijŅ©µź³öŹŪµÄ”°ĖŁĮ¦·Ę”±ŹĒ·ńŹ§Š§£¬Č”10.00gøĆŅ©Ę·Č«²æČÜÓŚĻ”ĮņĖį£¬ÅäÖĘ³É1000mLČÜŅŗ”£Č”ĘäÖŠ20.00mL£¬ÓĆ0.01000 mol/L KMnO4ČÜŅŗµĪ¶Ø£¬ÓĆČ„KMnO4ČÜŅŗ24.00mL”£Ķعż¼ĘĖćĖµĆ÷øĆŅ©ĪļŹĒ·ńÄÜ·žÓĆ£æ£ØMnO4£ŌŚĖįŠŌĢõ¼žĻĀµÄ»¹Ō²śĪļĪŖMn2£«£¬Ņ©Ę·ÖŠ³żFeŌŖĖŲĶāĘäĖū³É·Ö²»ÓėKMnO4·“Ó¦£©
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com