
Ӣ������������������ˡ����������ķ�����������ʹ�õ����ϴ����һ����һ���٣�������Ϊ������̬���������������Ⱦ��Ϊ�˷�����Ⱦ����һ����Ҫ��;���ǽ��ϳɸ߷��ӻ��������±��С���ӻ����Ŀǰ�Խṹ��ʽΪ��
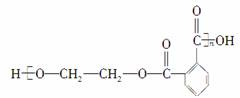 �������Ѿ��ɹ���ʵ�������ִ������Է�������CH3OH�����������ܵõ����л��������(����)
�������Ѿ��ɹ���ʵ�������ִ������Է�������CH3OH�����������ܵõ����л��������(����)
�١� ��������������HOCH2CH2OH
��������������HOCH2CH2OH
��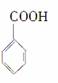 ����������
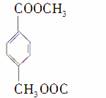
����������
A���٢� B���ڢ� C���ڢ� D���٢�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ӵ���У���Ҫһ���л��ۺ�����Ϊ������֮�������Ǩ�ƵĽ��ʣ����л��ۺ���ĵ���֮һ����G��ʾ���Ľṹ��ʽ���£�
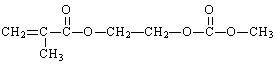 ������G�ĺϳɷ������£�
������G�ĺϳɷ������£�

��ش��������⣺��1����Ӧ�١��ݵķ�Ӧ���ͷֱ�Ϊ�� ������������ ������
��2��A�Ľṹ��ʽ������������������������
��3��д������-OH��-COOH��D��ͬ���칹������2�֣�
�� �������� ����
��4��д��B��C��Ӧ����ʽ ��
��5��д��E��F��Ӧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)�����ͬ��Ũ�Ⱦ�Ϊ0.2 mol��L��1�������CH3COOH��Һ���ֱ��ˮϡ��10������Һ��pH�ֱ���m��n����m��n�Ĺ�ϵΪ________��
(2)�����ͬ��Ũ�Ⱦ�Ϊ0.2 mol��L��1�������CH3COOH��Һ���ֱ��ˮϡ��m����n������Һ��pH�����3����m��n�Ĺ�ϵΪ________��
(3)�����ͬ��pH������1�������CH3COOH��Һ���ֱ��ˮϡ��m����n������Һ��pH�����3����m��n�Ĺ�ϵΪ________��
(4)�����ͬ��pH������13�İ�ˮ��NaOH��Һ���ֱ��ˮϡ��m����n������Һ��pH�����9����m��n�Ĺ�ϵΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳɲ��ϵ�Ӧ���뷢չ������ط��������ǵ�������ϳɲ��Ϸ�����ļ�������ȴ�����ˡ���ɫ��Ⱦ�����⣬���������ڡ���ɫ��Ⱦ���������(����)
�����ڷ��ա����㵹�뽭�Ӻ���֮�С��ܼ�ǿ������������߹�����ʶ���ݶ���������á��Ľ��䷽���������գ�����������������Ϣ߲�����������ʹ��������Ʒ��������������ʴ�����ԭΪС����
A���٢ڢ� B���ܢݢޢ�
C���� D���ڢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Է�������Ϊ94.5�ı����л���A(��C��H��O��Cl����Ԫ��)����һ�������¿��Է�����ͼ��ʾ��ת��(���������ˮ����ȥ)��
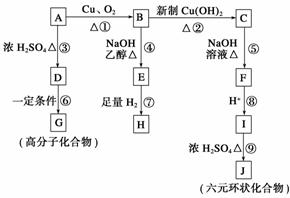
�ش��������⣺
(1)д���������ʵĽṹ��ʽ��
F��________________________________________________________________________��
H��________________________________________________________________________��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ��
A��B��________________________________________________________________________��
D��G��
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ۺ���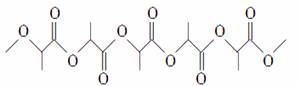 (�ṹ��ʽ)�ɱ��������գ�����Ϊ��Ʒ�������IJ��ϣ��������������������ʾۺ϶���(����)
(�ṹ��ʽ)�ɱ��������գ�����Ϊ��Ʒ�������IJ��ϣ��������������������ʾۺ϶���(����)
A��CH3CH(OH)COOH
B��HCOOCH2OH
C��HOCH2CH2COOH
D��HOCH(CH3)COOCH(CH3)CH2OH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
M5��ά�ǽ�������������һ�ֳ���������ά���������еķ����Ʋ�����35%�������Ƴɵ�ͷ�����������ĺ���ǰ������壬��Ϊ��װ��Ա�ṩ������������ϡ�������M5��ά�ĺϳ�·��(���ַ�Ӧδע������)��
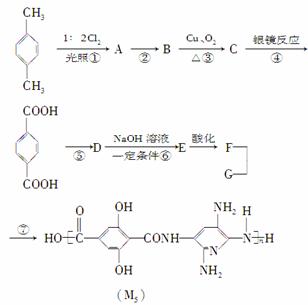
��֪������Ӧ����Ϊ��������X2��Ӧʱ��ͨ����X2�������������������ϵ���ԭ�ӷ���ȡ����Ӧ��������Ӧ����Ϊ������������X2��Ӧʱ��ͨ��Ϊ�����ϵ���ԭ��ֱ�ӱ�ȡ����
���������ϳ�M5��ά�Ĺ��̣�����������⣺
(1)�ϳ�M5��ά�ĵ���G�Ľṹ��ʽΪ
________________________________________________________________________
________________________________________________________________________��
F�ĺ��������ŵ�������
________________________________________________________________________��
(2)�ڢ١��ߵķ�Ӧ�У�������ȡ����Ӧ����______________���ڵķ�Ӧ������
________________________________________________________________________��
(3)����A��ͬʱ�������ɵ�A��ͬ���칹��Ϊ
________________________________________________________________________��
(4)1 mol��C���������Ƶ�������ͭ����Һ��Ӧ��������________ molש��ɫ������
(5)1 mol F��Na2CO3��Һ��Ӧ�������Na2CO3______mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�������ʾ��ͼһ�µ���

A��ͼ�ٱ�ʾ25��ʱ����0.1 mol��L��1����ζ�10 mL 0.05 mol��L��1 Ba(OH)2��Һ����Һ��pH�����������ı仯
B��ͼ�ڱ�ʾ����NO2�ĺ����ܱ�������tʱ������ѹǿʱ��c��NO2����ʱ��ı仯
C��ͼ�������߱�ʾ��ӦN2 (g) +3 H2(g)  2NH3(g) ��H < 0 �������淴Ӧ��ƽ�ⳣ��K���¶ȵı仯
2NH3(g) ��H < 0 �������淴Ӧ��ƽ�ⳣ��K���¶ȵı仯
D��ͼ����a��b���߷ֱ��ʾ��Ӧ2SO2(g) + O2(g)  2SO3(g) ��H<0ʹ�ã�a����δʹ�ã�b������ʱ����Ӧ�����е������仯
2SO3(g) ��H<0ʹ�ã�a����δʹ�ã�b������ʱ����Ӧ�����е������仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������£����и��������м���û�з�Ӧ���ǣ� ��
A�������������� B����������� C������������ D�����������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com