
”¾ĢāÄæ”æ¢ń.£Ø1£©25”ę 101 kPaŹ±£¬ĒāĘųŗĶŃõĘų·“Ӧɜ³É1molĖ®ÕōĘų·ÅČČ241.8kJ£¬øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ____”£
£Ø2£©Čō1gĖ®ÕōĘų×Ŗ»ÆĪŖŅŗĢ¬Ė®·ÅČČ2.444 kJ£¬Ōņ·“Ó¦2H2(g)£«O2(g)=2H2O(l)µÄ¦¤H£½___£¬ÓÉ“ĖæÉÖŖĒāĘųµÄČ¼ÉÕČČĪŖ____”£(½į¹ū±£ĮōŠ”ŹżµćŗóŅ»Ī»)
¢ņ.ŅŃÖŖH£«(aq)£«OH£(aq)=H2O(l) ¦¤H£½£57.3kJ/mol£¬»Ų“šĻĀĮŠÓŠ¹ŲÖŠŗĶ·“Ó¦µÄĪŹĢā£ŗ
£Ø1£©ÓĆ0.1molBa(OH)2Åä³ÉĻ”ČÜŅŗÓė×ćĮæĻ”ĻõĖį·“Ó¦£¬Äܷųö___kJµÄÄÜĮ攣
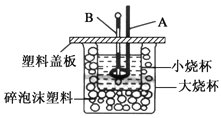
£Ø2£©ČēĶ¼ĖłŹ¾×°ÖĆÖŠ£¬ŅĒĘ÷AµÄĆū³ĘŹĒ___£¬×÷ÓĆŹĒ__£»ĖéÅŻÄĖÜĮĻµÄ×÷ÓĆŹĒ___”£
£Ø3£©ĶعżŹµŃé²ā¶ØµÄÖŠŗĶČȵĦ¤H³£³£“óÓŚ£57.3kJ/mol£¬ĘäŌŅņæÉÄÜŹĒ___”£
£Ø4£©ÓĆĻąĶ¬ÅضČŗĶĢå»żµÄ°±Ė®(NH3”¤H2O)“śĢęNaOHČÜŅŗ½ųŠŠÉĻŹöŹµŃ飬²āµĆµÄÖŠŗĶČȵďżÖµ____(Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°ĪŽÓ°Ļģ”±)”£
”¾“š°ø”æH2(g)£«![]() O2(g)=H2O(g) ¦¤H£½£241.8 kJ/mol £571.6kJ/mol 285.8kJ/mol 11.46 »·ŠĪ²£Į§½Į°č°ō ½Į°č£¬Ź¹ČÜŅŗ³ä·Ö»ģŗĻ ±£ĪĀ”¢øōČČ”¢¼õÉŁŹµŃé¹ż³ĢÖŠµÄČČĮæĖšŹ§ ŹµŃéÖŠ²»æɱÜĆāÓŠÉŁĮæČČĮæĖšŹ§ Ę«Š”
O2(g)=H2O(g) ¦¤H£½£241.8 kJ/mol £571.6kJ/mol 285.8kJ/mol 11.46 »·ŠĪ²£Į§½Į°č°ō ½Į°č£¬Ź¹ČÜŅŗ³ä·Ö»ģŗĻ ±£ĪĀ”¢øōČČ”¢¼õÉŁŹµŃé¹ż³ĢÖŠµÄČČĮæĖšŹ§ ŹµŃéÖŠ²»æɱÜĆāÓŠÉŁĮæČČĮæĖšŹ§ Ę«Š”
”¾½āĪö”æ
¢ń.(1)øł¾ŻĒāĘųŗĶŃõĘų·“Ӧɜ³É1molĖ®ÕōĘų·ÅČČ241.8kJ£¬ŹéŠ“·“Ó¦µÄČČ»Æѧ·“Ó¦·½³ĢŹ½£»
(2)øł¾Ż1gĖ®ÕōĘų×Ŗ»Æ³ÉŅŗĢ¬Ė®·ÅČČ2.444kJ£¬¼ĘĖć1molĖ®ÕōĘų×Ŗ»ÆĪŖŅŗĢ¬Ė®·Å³öµÄČČĮ棬ŌŁøł¾ŻøĒĖ¹¶ØĀɼĘĖćĘäģŹ±ä£»
¢ņ. (1)ÓÉH+(aq)+OH-(aq)ØTH2O(l)”÷H=-57.3kJmol-1æÉÖŖÉś³É1molH2O·Å³öČČĮæĪŖ57.3kJ£¬Č»ŗóøł¾ŻĖ®µÄĪļÖŹµÄĮæÓėČČĮæ³ÉÕż±ČĒó³öČČĮ棻
(2)øł¾ŻĶ¼Ź¾ÅŠ¶ĻŅĒĘ÷Ćū³Ę£»ÖŠŗĶČČ²ā¶ØŹ±Ó¦¾”Įæ¼õÉŁČČĮæÉ¢Ź§£»
(3)Źµ¼ŹŹµŃé¹ż³ĢÖŠŅ»¶ØÓŠČČĮæÉ¢Ź§£»
(4)NH3H2OĪŖČõµē½āÖŹ£¬µēĄėŠčŅŖĪüČČ£¬¾Ż“Ė·ÖĪöÅŠ¶Ļ”£
¢ń.(1)ÓÉĒāĘųŗĶŃõĘų·“Ӧɜ³É1molĖ®ÕōĘų·ÅČČ241.8kJ£¬Éś³É2molĖ®ÕōĘų·Å³öČČĮæĪŖ483.6kJ£¬øĆ·“Ó¦ČČ»Æѧ·“Ó¦·½³ĢŹ½ĪŖH2(g)£«![]() O2(g)=H2O(g) ¦¤H£½£241.8 kJ/mol»ņ2H2(g)+O2(g)=2H2O(g)”÷H=-483.6kJ/mol£¬¹Ź“š°øĪŖ£ŗH2(g)£«
O2(g)=H2O(g) ¦¤H£½£241.8 kJ/mol»ņ2H2(g)+O2(g)=2H2O(g)”÷H=-483.6kJ/mol£¬¹Ź“š°øĪŖ£ŗH2(g)£«![]() O2(g)=H2O(g) ¦¤H£½£241.8 kJ/mol»ņ2H2(g)+O2(g)=2H2O(g)”÷H=-483.6kJ/mol£»
O2(g)=H2O(g) ¦¤H£½£241.8 kJ/mol»ņ2H2(g)+O2(g)=2H2O(g)”÷H=-483.6kJ/mol£»
(2)Čō1gĖ®ÕōĘų×Ŗ»Æ³ÉŅŗĢ¬Ė®·ÅČČ2.444kJ£¬Ōņ1molĖ®ÕōĘų×Ŗ»ÆĪŖŅŗĢ¬Ė®·Å³öČČĮæ= =43.992kJ/mol£¬øł¾ŻøĒĖ¹¶ØĀÉÖŖĘäģŹ±ä=-483.6kJ/mol+(-43.992kJ/mol)”Į2=-571.58kJ/mol”Ö-571.6kJ/mol£¬ŌņĒāĘųµÄČ¼ÉÕČČ=
=43.992kJ/mol£¬øł¾ŻøĒĖ¹¶ØĀÉÖŖĘäģŹ±ä=-483.6kJ/mol+(-43.992kJ/mol)”Į2=-571.58kJ/mol”Ö-571.6kJ/mol£¬ŌņĒāĘųµÄČ¼ÉÕČČ=![]() =285.8kJ/mol£¬¹Ź“š°øĪŖ£ŗ-571.6kJ/mol£»285.8kJ/mol£»
=285.8kJ/mol£¬¹Ź“š°øĪŖ£ŗ-571.6kJ/mol£»285.8kJ/mol£»
¢ņ.(1)ÓÉH+(aq)+OH-(aq)ØTH2O(l)”÷H=-57.3kJmol-1æÉÖŖÉś³É1molH2O·Å³öČČĮæĪŖ57.3kJ£¬0.1mol Ba(OH)2Åä³ÉĻ”ČÜŅŗÓė×ćĮæĻ”ĻõĖį·“Ó¦æɵĆ0.2molH2O£¬ĖłŅŌ·Å³öµÄČČĮæĪŖ57.3kJ”Į0.2=11.46kJ£¬¹Ź“š°øĪŖ£ŗ11.46£»
(2)øł¾ŻĶ¼Ź¾£¬ŅĒĘ÷AŹĒ»·ŠĪ²£Į§½Į°č°ō£¬æÉĘšµ½½Į°č£¬Ź¹ČÜŅŗ³ä·Ö»ģŗĻµÄÄæµÄ£»ÖŠŗĶČČ²ā¶ØŹµŃé³É°ÜµÄ¹Ų¼üŹĒ±£ĪĀ¹¤×÷£¬“óŠ”ÉÕ±Ö®¼äĢīĀśĖéÅŻÄĖÜĮĻæÉŅŌĘšµ½±£ĪĀ”¢øōČČ£¬¼õÉŁČČĮæÉ¢Ź§µÄ×÷ÓĆ£¬¹Ź“š°øĪŖ£ŗ»·ŠĪ²£Į§½Į°č°ō£»½Į°č£¬Ź¹ČÜŅŗ³ä·Ö»ģŗĻ£»±£ĪĀ”¢øōČČ£¬¼õÉŁŹµŃé¹ż³ĢÖŠµÄČČĮæÉ¢Ź§£»
(3) Źµ¼ŹŹµŃé¹ż³ĢÖŠŅ»¶ØÓŠ²æ·ÖČČĮæÉ¢Ź§£¬Ņņ“ĖĒóµĆµÄÖŠŗĶČČ”÷H“óÓŚ-57.3kJmol-1£¬¹Ź“š°øĪŖ£ŗŹµŃé¹ż³ĢÖŠ²»æɱÜĆāÓŠČČĮæÉ¢Ź§£»
(4)NH3H2OĪŖČõ¼ī£¬µēĄė¹ż³ĢĪŖĪüČČ¹ż³Ģ£¬ÓĆĻąĶ¬ÅضČŗĶĢå»żµÄ°±Ė®(NH3H2O)“śĢęNaOHČÜŅŗ½ųŠŠÉĻŹöŹµŃ飬²āµĆµÄÖŠŗĶČȵďżÖµĘ«Š”£¬¹Ź“š°øĪŖ£ŗĘ«Š””£


 ĘŚÄ©±¦µäµ„ŌŖ¼ģ²ā·ÖĄąø“Ļ°¾ķĻµĮŠ“š°ø
ĘŚÄ©±¦µäµ„ŌŖ¼ģ²ā·ÖĄąø“Ļ°¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŅŃÖŖAĪŖµ»ĘÉ«¹ĢĢ壬RŹĒµŲæĒÖŠŗ¬Įæ×ī¶ąµÄ½šŹōŌŖĖŲµÄµ„ÖŹ£¬TĪŖÉś»īÖŠŹ¹ÓĆ×ī¹ć·ŗµÄ½šŹōµ„ÖŹ£¬DŹĒ¾ßÓŠ“ÅŠŌµÄŗŚÉ«¾§Ģå £¬C”¢FŹĒĪŽÉ«ĪŽĪ¶µÄĘųĢ壬HŹĒ°×É«³Įµķ£¬WČÜŅŗÖŠµĪ¼ÓKSCNČÜŅŗ³öĻÖŗģÉ«”£
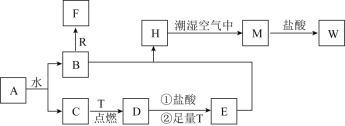
(1)ĪļÖŹDµÄ»ÆѧŹ½ĪŖ___________£¬HµÄĆū³Ę___________”£
(2)![]() ŌŚ³±ŹŖæÕĘųÖŠ±ä³ÉMµÄŹµŃéĻÖĻóŹĒ______________£¬»Æѧ·½³ĢŹ½ĪŖ___________”£
ŌŚ³±ŹŖæÕĘųÖŠ±ä³ÉMµÄŹµŃéĻÖĻóŹĒ______________£¬»Æѧ·½³ĢŹ½ĪŖ___________”£
(3)![]() ÓėWČÜŅŗŅ²ÄÜ·¢Éś·“Ó¦£¬Ęä·“Ó¦µÄĄąŠĶĪŖ______(ĢīŠņŗÅ)”£
ÓėWČÜŅŗŅ²ÄÜ·¢Éś·“Ó¦£¬Ęä·“Ó¦µÄĄąŠĶĪŖ______(ĢīŠņŗÅ)”£
![]() »ÆŗĻ·“Ó¦
»ÆŗĻ·“Ó¦ ![]() ÖĆ»»·“Ó¦
ÖĆ»»·“Ó¦ ![]() ø“·Ö½ā·“Ó¦
ø“·Ö½ā·“Ó¦ ![]() Ńõ»Æ»¹Ō·“Ó¦
Ńõ»Æ»¹Ō·“Ó¦
(4)![]() ŗĶRŌŚČÜŅŗÖŠ·“Ӧɜ³ÉFµÄĄė×Ó·½³ĢŹ½ĪŖ____________________”£
ŗĶRŌŚČÜŅŗÖŠ·“Ӧɜ³ÉFµÄĄė×Ó·½³ĢŹ½ĪŖ____________________”£
(5)½«![]() Ķ¶Čėµ½EČÜŅŗÖŠ£¬æÉŅŌ¹Ū²ģµ½µÄĻÖĻóŹĒ£ŗ___________________”£
Ķ¶Čėµ½EČÜŅŗÖŠ£¬æÉŅŌ¹Ū²ģµ½µÄĻÖĻóŹĒ£ŗ___________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻū³żĪ²ĘųÖŠµÄNOŹĒ»·¾³æĘѧъ¾æµÄČȵćæĪĢā”£
I£®NOŃõ»Æ»śĄķ
ŅŃÖŖ£ŗ2NO(g)+O2(g) ![]() 2NO2(g)”÷H=-110kJ”¤mol-1
2NO2(g)”÷H=-110kJ”¤mol-1
25”ꏱ£¬½«NOŗĶO2°“ĪļÖŹµÄĮæÖ®±ČĪŖ2:1³äČėŗćČŻ·“ӦȯĘ÷ÖŠ£¬ÓĆ²āŃ¹·ØŃŠ¾æĘä·“Ó¦µÄ½ųŠŠĒéæö”£ĢåĻµµÄ×ÜŃ¹ĒæpĖꏱ¼ätµÄ±ä»ÆČēĻĀ±ķĖłŹ¾(ŗöĀŌNO2ÓėN2O4µÄ×Ŗ»Æ)
t/min | 0 | 80 | 160 |
|
p/kPa | 75.0 | 63.0 | 55.0 | 55.0 |
£Ø1£©0~80min£¬v(O2)=_____kPa/min£»Ėę×Å·“Ó¦½ųŠŠ£¬·“Ó¦ĖŁĀŹÖš½„¼õŠ”µÄŌŅņŹĒ______________”£
ÓĆĘ½ŗā·ÖŃ¹“śĢęĘ½ŗāÅضČĖłµĆµ½µÄĘ½ŗā³£ŹżÓĆK(p)±ķŹ¾£¬25”ꏱ£¬K(p)µÄÖµĪŖ_______(±£Įō3Ī»ÓŠŠ§Źż×Ö)”£
£Ø2£©²éŌÄ׏ĮĻ£¬¶ŌÓŚ×Ü·“Ó¦2NOg)+O2(g) ![]() 2NO2(g)ÓŠČēĻĀĮ½²½Ąś³Ģ
2NO2(g)ÓŠČēĻĀĮ½²½Ąś³Ģ
µŚŅ»²½2NO(g) ![]() N2O2(g) æģĖŁ·“Ó¦
N2O2(g) æģĖŁ·“Ó¦
µŚ¶ž²½N2O2(g)+O2(g) ![]() 2NO2(g) Āż·“Ó¦
2NO2(g) Āż·“Ó¦
×Ü·“Ó¦ĖŁĀŹÖ÷ŅŖÓɵŚ_____²½¾ö¶Ø£»ČōĄūÓĆ·Ö×Ó²¶»ńĘ÷ŹŹµ±¼õÉŁ·“ӦȯĘ÷ÖŠµÄN2O2£¬×Ü·“Ó¦µÄĘ½ŗā³£ŹżK(p)½«___(Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±)£»ČōĢįøß·“Ó¦ĪĀ¶ČÖĮ35”ę£¬ŌņĢåĻµŃ¹ĒæP(35”ę)______P(25”ę)(Ģī”°“óÓŚ”±”¢”°µČÓŚ”±»ņ”°Š”ÓŚ”±)”£
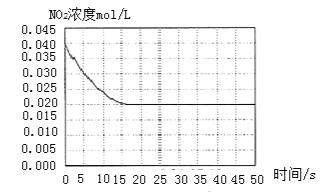
II. ijĪĀ¶ČĻĀŅ»ĆܱÕČŻĘ÷ÖŠ³äČėŅ»¶ØĮæµÄNO2£¬²āµĆNO2ÅضČĖꏱ¼ä±ä»ÆµÄĒśĻßČēÉĻĶ¼ĖłŹ¾”£
£Ø1£©·“Ó¦ĢåĻµ“ļĘ½ŗāŗóŃ¹ĒæĪŖP1£¬ČōÉżøßĪĀ¶Č£¬ŌŁ“Ī“ļĘ½ŗāŗ󣬻ģŗĻĘųĢåµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæ______![]() Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±
Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±![]() £»
£»
£Ø2£©ČōŌŚŗćĪĀŗćČŻĢõ¼žĻĀ£¬ĻņĘ½ŗāĢåĻµÖŠ³äČėŅ»¶ØĮæO2£¬ŌŁ“Ī“ļĘ½ŗāŗ󣬲āµĆŃ¹ĒæĪŖP2£¬c(O2)=0.09mol/L£¬ŌņP2£ŗP1=______
£Ø3£©øĆĪĀ¶ČĻĀ·“Ó¦2NO(g)+O2(g) ![]() 2NO2(g)µÄ»ÆŃ§Ę½ŗā³£ŹżKĪŖ______”£
2NO2(g)µÄ»ÆŃ§Ę½ŗā³£ŹżKĪŖ______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ³£ĪĀĻĀ£¬ŌŚpH=5µÄCH3COOHČÜŅŗÖŠ“ęŌŚČēĻĀµēĄėĘ½ŗā£ŗCH3COOH![]() CH3COO-+H+£¬¶ŌÓŚøĆĘ½ŗā£¬ĻĀĮŠŠšŹöÕżČ·µÄŹĒ
CH3COO-+H+£¬¶ŌÓŚøĆĘ½ŗā£¬ĻĀĮŠŠšŹöÕżČ·µÄŹĒ
A£®¼ÓČėĖ®Ź±£¬Ę½ŗāĻņÓŅŅĘ¶Æ£¬CH3COOHµēĄė³£ŹżŌö“ó
B£®¼ÓČėÉŁĮæCH3COONa¹ĢĢå£¬Ę½ŗāĻņÓŅŅʶÆ
C£®¼ÓČėÉŁĮæNaOH¹ĢĢå£¬Ę½ŗāĻņÓŅŅĘ¶Æ£¬c(H+)¼õŠ”
D£®¼ÓČėÉŁĮæpH=5µÄĮņĖį£¬ČÜŅŗÖŠc(H+)Ōö“ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ£ØŅ»£©ŹµŃéŹŅÓĆĶ¼¼××°ÖĆÖʱøSO3²¢²ā¶ØSO2“ß»ÆŃõ»ÆĪŖSO3µÄ×Ŗ»ÆĀŹ”£
ŅŃÖŖ£ŗSO3ČŪµćĪŖ16.8”ę£¬·ŠµćĪŖ44.8”ę£¬¼ŁÉčĘųĢå½ųČė×°ÖĆæÉŅŌ±»ĶźČ«ĪüŹÕ£¬²»æ¼ĀĒæÕĘųµÄÓ°Ļģ”£

£Ø1£©AÖŠŹ¹ÓĆÅØĮņĖįµÄÖŹĮæ·ÖŹżĪŖ70%µÄŌŅņŹĒ___£¬BÖŠÅØH2SO4µÄ×÷ÓĆŹĒ___”£
£Ø2£©µ±ŹµŃéĶ£Ö¹ĶØČėSO2£¬ĻØĆš¾Ę¾«µĘŗ󣬻¹ŠčŅŖ¼ĢŠųĶØŅ»¶ĪŹ±¼äµÄŃõĘų£¬ĘäÄæµÄŹĒ___”£
£Ø3£©ŹµŃé½įŹųŗó²āµĆ×°ÖĆDŌö¼ÓĮĖag£¬×°ÖĆEÖŠµÄ³ĮµķĻ“µÓŗęøÉŗóĘäÖŹĮæĪŖbg”£ŌņEÖŠµÄ³ĮµķµÄ»Æѧ³É·ÖŹĒ___£ØĢīŠ“»ÆѧŹ½£©£¬±¾ŹµŃéÖŠSO2×Ŗ»ÆĀŹĪŖ___£ØÓĆ“śŹżŹ½±ķŹ¾£¬²»ÓĆ»Æ¼ņ£©”£
£Ø¶ž£©SO3ČÜÓŚÅØĮņĖįŗóæɵƵ½·¢ŃĢĮņĖį£¬¹¤ŅµÉĻ°ŃøÉŌļµÄĀČ»ÆĒāĘųĢåĶØČėµ½·¢ŃĢĮņĖįÖŠæÉŅŌµĆµ½HSO3Cl”£HSO3ClŹĒŅ»ÖÖĪŽÉ«ŅŗĢ壬·ŠµćĪŖ152”ę£¬ÓŠĒæøÆŹ“ŠŌ£¬ÓöŹŖæÕĘų²śÉśĒæĮŅµÄ°×Īķ”£ĻÖÓĆĶ¼ŅŅĖłŹ¾µÄ×°ÖĆÖĘČ”HSO3Cl£Ø¼Š³Ö¼°¼ÓČČ×°ÖĆĀŌČ„£©”£

£Ø1£©HSO3ClÓöŹŖæÕĘų²śÉśĒæĮŅµÄ°×Īķ£¬Ēė½įŗĻÓĆ»Æѧ·½³ĢŹ½½āŹĶĘäŌŅņ___”£
£Ø2£©·ÖŅŗĀ©¶·ĻĀ·½½ÓµÄĆ«Ļø¹Ü£¬Ęä×÷ÓĆŹĒ___£»Čō²»ÓĆĆ«Ļø¹Ü¶ųÖ±½ÓÓĆ·ÖŅŗĀ©¶·×¢ČėÅØŃĪĖį£¬æÉÄÜ·¢ÉśµÄĻÖĻóŹĒ___”£
£Ø3£©×°ÖĆFµÄ×÷ÓĆŹĒ___”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĪŖĢ½¾æijÄŃČÜŠŌŃĪX(½öŗ¬ČżÖÖ³£¼ūŌŖĖŲ)µÄ×é³É£¬Éč¼Ę²¢Ķź³ÉŅŌĻĀŹµŃé(Į÷³ĢÖŠ²æ·ÖĪļÖŹŅŃĀŌČ„)£ŗ
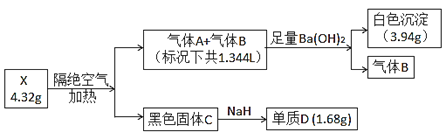
ŅŃÖŖ£ŗĘųĢåAŗĶĘųĢåBĖłŗ¬ŌŖĖŲĻąĶ¬£¬¶¼ŹĒĪŽÉ«ĪŽĪ¶ĘųĢ壬¹ĢĢåCĪŖ“æ¾»ĪļĒŅ¾ßÓŠ“ÅŠŌ£¬µ„ÖŹDŹĒÄæĒ°½ØÖžŠŠŅµÓ¦ÓĆ×ī¹ć·ŗµÄ½šŹō”£øł¾ŻÉĻŹöŠÅĻ¢£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ŃĪXµÄ»ÆѧŹ½ĪŖ________________”£
(2)ĪŽĖ®Ģõ¼žĻĀ£¬ÉŁĮæNaH¾ĶÄÜÓė¹ĢĢåC·“Ó¦²¢·Å³ö“óĮæµÄČČ£¬Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½___________________”£
(3)½«²śÉśµÄĘųĢåAČ«²æ±»100 mL 0.35 mol”¤L£1ĒāŃõ»ÆÄĘČÜŅŗ³ä·ÖĪüŹÕ£¬·“Ó¦µÄ×ÜĄė×Ó·½³ĢŹ½ĪŖ____________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠ·“Ó¦ŹōÓŚ·ĒŃõ»Æ»¹Ō·“Ó¦µÄŹĒ£Ø””””£©
A. Fe2O3+3CO![]() 2Fe+3CO2 B. NH4NO3
2Fe+3CO2 B. NH4NO3![]() N2Oӟ+2H2O
N2Oӟ+2H2O
C. 2NaHCO3![]() Na2CO3+CO2”ü+H2O D. CuO+COØTCu+CO2
Na2CO3+CO2”ü+H2O D. CuO+COØTCu+CO2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĶ¼ŹĒĮņĖįŹŌ¼ĮĘæ±źĒ©ÉĻµÄÄŚČŻ£ŗ

(1)ij»ÆѧŠĖȤŠ”×é½ųŠŠĮņĖįŠŌÖŹµÄŹµŃéĢ½¾æŹ±£¬ŠčŅŖ490mL 4.6mol”¤L-1µÄĻ”ĮņĖį£¬ĻÖŅŖÅäÖĘøĆÅØ¶ČµÄČÜŅŗĖłŠčµÄ²£Į§ŅĒĘ÷³żĮæĶ²”¢ÉÕ±”¢²£Į§°ō”¢½ŗĶ·µĪ¹ÜĶā£¬»¹ŠčŅŖ__________(ĢīŅĒĘ÷Ćū³Ę)£»ŠčŅŖĮæČ”98%ÅØĮņĖį____________mL½ųŠŠÅäÖĘ£»
(2)ÅäÖĘČÜŅŗŹ±ÓŠČēĻĀ²Ł×÷£ŗa.Ļ”ŹĶČܽāb.Ņ”ŌČc.Ļ“µÓd.ĄäČ“e.ĮæČ”f.½«ČÜŅŗŅĘÖĮČŻĮæĘæg.¶ØČŻ£¬ŹµŃé²Ł×÷Ė³ŠņÕżČ·µÄŹĒ£Ø___________£©”£
A. e”śa”śf”śd”śc”śf”śg”śb B. e”śa”śd”śf”śc”śf”śg”śb
C. e”śa”śf”śd”śc”śf”śb”śg D. e”śa”śd”śf”śc”śf”śb”śg
(3)ĻĀĮŠĪŖÅäÖĘ¹ż³ĢÖŠ²æ·Ö²Ł×÷µÄŹ¾ŅāĶ¼£¬ĘäÖŠÓŠ“ķĪóµÄŹĒ____(ĢīŠņŗÅ)£»

(4)ŌŚÅäÖĘ4.6mol”¤L-1Ļ”ĮņĖįµÄ¹ż³ĢÖŠ£¬ĻĀĮŠĒéæö»įŅżĘšÅäÖĘĖłµĆµÄĮņĖįČÜŅŗĪļÖŹµÄĮæÅضČĘ«øߵďĒ___£»
A.Ī“¾ĄäČ“³ĆČČ½«ČÜŅŗ×¢ČėČŻĮæĘæÖŠ B.ČŻĮæĘæĻ“µÓŗó£¬Ī“øÉŌļ“¦Ąķ
C.¶ØČŻŹ±ŃöŹÓ¹Ū²ģŅŗĆę D.Ī“Ļ“µÓÉÕ±ŗĶ²£Į§°ō
(5)ĪŖÖŠŗĶ100mL 2.3 mol”¤L-1KOHČÜŅŗŗóĻŌÖŠŠŌ£¬ŠčŅŖ¼ÓČė________mL 4.6mol”¤L-1Ļ”ĮņĖį”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ25”ę”¢101kPaĻĀ£¬Ģ¼”¢ĒāĘų”¢¼×ĶéŗĶĘĻĢŃĢĒµÄČ¼ÉÕČČŅĄ“ĪŹĒ393.5kJ/mol”¢285.8kJ/mol”¢890.3kJ/mol”¢2800kJ/mol£¬ŌņĻĀĮŠČČ»Æѧ·½³ĢŹ½ÕżČ·µÄŹĒ
A.C£Øs£©+![]() O2£Øg£©£½CO£Øg£© ¦¤H=-393.5kJ/mol
O2£Øg£©£½CO£Øg£© ¦¤H=-393.5kJ/mol
B.2H2£Øg£©+O2£Øg£©£½2H2O£Øl£© ¦¤H =+571.6kJ/mol
C.CH4£Øg£©+2O2£Øg£©£½ CO2£Øg£©+2H2O£Øg£© ¦¤H=-890.3kJ/mol
D.C6H12O6£Øs£©+6O2£Øg£©£½6CO2£Øg£©+6H2O£Øl£© ¦¤H=-2800kJ/mol
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com