
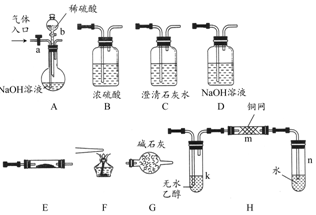
 ČēĶ¼ĖłŹ¾ĪŖ³£¼ūĘųĢåÖʱø”¢·ÖĄė”¢øÉŌļŗĶŠŌÖŹŃéÖ¤µÄ²æ·ÖŅĒĘ÷×°ÖĆ£Ø¼ÓČČÉč±ø¼°¼Š³Ö¹Ģ¶Ø×°ÖĆ¾łĀŌČ„£©£¬Ēėøł¾ŻŅŖĒóĶź³ÉĻĀĮŠø÷Ģā£ØŅĒĘ÷×°ÖĆæÉČĪŅāŃ”ÓĆ£¬±ŲŅŖŹ±æÉÖŲø“Ń”Ōń£©£®
ČēĶ¼ĖłŹ¾ĪŖ³£¼ūĘųĢåÖʱø”¢·ÖĄė”¢øÉŌļŗĶŠŌÖŹŃéÖ¤µÄ²æ·ÖŅĒĘ÷×°ÖĆ£Ø¼ÓČČÉč±ø¼°¼Š³Ö¹Ģ¶Ø×°ÖĆ¾łĀŌČ„£©£¬Ēėøł¾ŻŅŖĒóĶź³ÉĻĀĮŠø÷Ģā£ØŅĒĘ÷×°ÖĆæÉČĪŅāŃ”ÓĆ£¬±ŲŅŖŹ±æÉÖŲø“Ń”Ōń£©£®·ÖĪö £Ø1£©ÓĆNaOHČÜŅŗ³ĪĒåŹÆ»ŅĖ®³żČ„CO2£¬ÓĆÅØĮņĖįøÉŌļCO£¬ÓĆCuOŃõ»ÆCO£¬ÓĆ³ĪĒåŹÆ»ŅĖ®¼ģŃéCOµÄŃõ»Æ²śĪļ£¬Č¼ÉշسżČ„¶ąÓąµÄCO£»
£Ø2£©Ķ£Ö¹COŗĶCO2»ģŗĻĘųĢåµÄĶØČė£¬aÓ¦¹Ų±Õ£¬b“ņæŖ£¬ÅØĮņĖįÓėNaOHČÜŅŗ·“Ó¦·Å³öČČĮ棬¼ÓæģAÖŠµÄĖ®µÄÕō·¢£¬H2OÓėEÖŠNa2O2·“Ó¦æÉÉś³ÉO2£¬¼ÓČČkÓŠĄūÓŚCH3CH2OHµÄ»Ó·¢£¬¼ÓČČm£¬CH3CH2OHÓėO2·“Ӧɜ³ÉCH3CHO£®
½ā“š ½ā£ŗ£Ø1£©ŅŖ»ńµĆ“æ¾»øÉŌļµÄCO¾Ķ±ŲŠėÓĆAÖŠµÄNaOHČÜŅŗĪüŹÕCO2£¬²¢ĶعżCÖŠµÄ³ĪĒåŹÆ»ŅĖ®²»±ä»ėÖ¤Ć÷CO2Ņѱ»ĶźČ«ĪüŹÕ£¬ŌŁĶعżBÅØĮņĖįøÉŌļCOĘųĢ壮COĶعżEÖŠ¼ÓČȵÄCuO±»Ńõ»Æ³ÉCO2£¬±»CÖŠ³ĪĒåŹÆ»ŅĖ®ĪüŹÕ±ä»ė×Ē£¬Ö¤Ć÷CO»¹ŌŠŌ¼°Ńõ»Æ²śĪļ£®ĖłŃ”×°ÖƵÄĮ¬½ÓĖ³ŠņĪŖACBECF£¬
¹Ź“š°øĪŖ£ŗACBECF£»ABÖ®¼äµÄC×°ÖĆÖŠČÜŅŗ±£³Ö³ĪĒ壬EFÖ®¼äµÄC×°ÖĆÖŠČÜŅŗ±ä»ė×Ē£»
£Ø2£©Ķ£Ö¹COŗĶCO2»ģŗĻĘųĢåµÄĶØČė¾ĶŅŖ¹Ų±Õ»īČūa£¬“ņæŖ»īČūb·ÅČėĻ”H2SO4ÓėNaOH ·¢ÉśÖŠŗĶ·“Ó¦£¬·ÅČČÓŠĖ®ÕōĘų“ÓA×°ÖĆÖŠ³öĄ“£¬ÓėEÖŠNa2O2·“Ó¦¾Ķ»įÓŠO2Éś³É£¬ø±²śĪļNaOH½ųČėDÖŠ£¬O2ŅŌBÖŠÅØH2SO4øÉŌļŌŁ½ųČėH×°ÖĆ½«ŅŅ“¼ÕōĘųÓėO2ÄÜŹŲ¼ÓČȵÄĶĖæĶų±»Ńõ»Æ³ÉŅŅČ©£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ2CH3CH2OH+O2$”ś_{”÷}^{Cu}$2CH3CHO+2H2O£»
¹Ź“š°øĪŖ£ŗ¹Ų±Õ£»“ņæŖ£»k”¢m£»2CH3CH2OH+O2$”ś_{”÷}^{Cu}$2CH3CHO+2H2O£®
µćĘĄ ±¾Ģāæ¼²éĮĖĘųĢåµÄÖʱø”¢·ÖĄė”¢øÉŌļŗĶŠŌÖŹŃéÖ¤”¢ŅŌ¼°ŅŅ“¼µÄĶŃĒāŃõ»Æ·“Ó¦µČ£¬ĢāÄæÄѶČÖŠµČ£¬×¢Ņā°ŃĪÕĘųĢåµÄÖʱø·½·ØŗĶŹµŃéŌĄķ£¬²ąÖŲÓŚæ¼²éѧɜµÄ·ÖĪöÄÜĮ¦ŗĶŹµŃéĢ½¾æÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
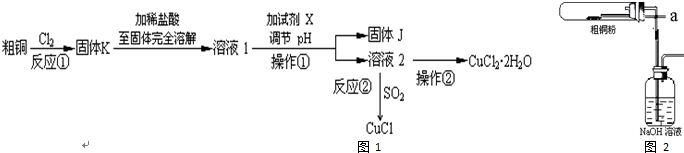
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
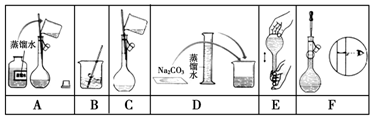
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | øıäµÄĢõ¼ž | ½įĀŪ |
| A | ÉżĪĀ | CO32-µÄĖ®½āĘ½ŗāĻņÓŅŅĘ¶Æ |
| B | ¼ÓČėAlCl3¹ĢĢå | ²śÉś“óĮæĘųĢå |
| C | ¼ÓČė100mLH2O | ČÜŅŗÖŠc£ØH+£©”¢c£ØOH-£©¾ł¼õŠ” |
| D | ¼ÓČėÉŁĮæCH3COONa¹ĢĢå | ČÜŅŗÖŠn£ØCO32-£©Ōö“ó |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Na0H¹ĢĢåŌŚæÕĘųÖŠ±äÖŹ | B£® | øÖĢśÉśŠā | ||
| C£® | ĪļÖŹµÄČ¼ÉÕ | D£® | Ö²ĪļµÄ¹āŗĻ×÷ÓĆ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
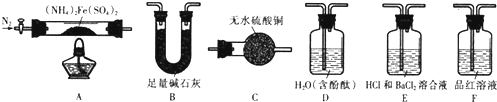
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
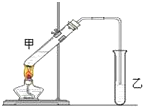 Ė×»°Ėµ£ŗ”°³Ā¾ĘĄĻ“×ĢŲ±šĻć”±£¬ĘäŌŅņŹĒ¾ĘŌŚ“ę“¢¹ż³ĢÖŠÉś³ÉĮĖÓŠĻćĪ¶µÄŅŅĖįŅŅõ„£¬ŌŚŹµŃéŹŅĄļĪŅĆĒŅ²æÉŅŌÓĆČēĶ¼ĖłŹ¾×°ÖĆĄ“Ä£ÄāøĆ¹ż³Ģ£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
Ė×»°Ėµ£ŗ”°³Ā¾ĘĄĻ“×ĢŲ±šĻć”±£¬ĘäŌŅņŹĒ¾ĘŌŚ“ę“¢¹ż³ĢÖŠÉś³ÉĮĖÓŠĻćĪ¶µÄŅŅĖįŅŅõ„£¬ŌŚŹµŃéŹŅĄļĪŅĆĒŅ²æÉŅŌÓĆČēĶ¼ĖłŹ¾×°ÖĆĄ“Ä£ÄāøĆ¹ż³Ģ£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com