
 �� ��
�� �� 1000mL����ƿ D ������
1000mL����ƿ D ������  �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��500mL 0.1mol��L��1 CuSO4��Һ������������գ�
��500mL 0.1mol��L��1 CuSO4��Һ������������գ�  �����������ƣ���
�����������ƣ���
 Ӧ��������
Ӧ�������� ƽ��
ƽ�� ȡ g��ˮCuSO4��
ȡ g��ˮCuSO4�� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 l2��2H2SO4
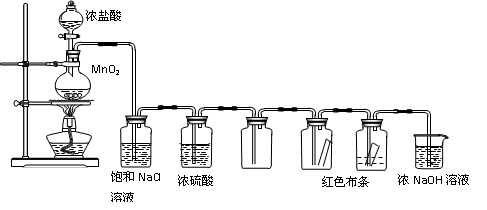
l2��2H2SO4 2CaSO4��2Cl2����2H2O�����������ͼ��ʾװ����ȡ��������֤�����ʵ�ʵ�顣
2CaSO4��2Cl2����2H2O�����������ͼ��ʾװ����ȡ��������֤�����ʵ�ʵ�顣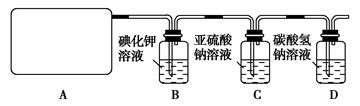
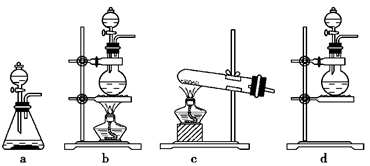
 ________________ ��
________________ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
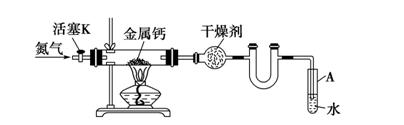
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
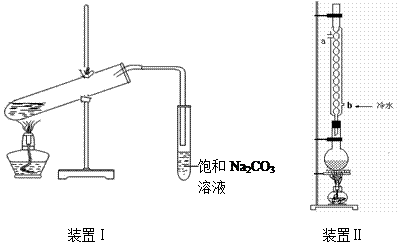
| �� �� |
| �е�/�� | �ܶ�/g��cm��3 | ||
| �� �� | ��114 | 78 | 0.789 | ||
| �� �� | 16.6 | 117.9 | 1.05 | ||
| �������� | ��83.6 | 77.5 | 0.900 | ||
| 98%H2SO4 | 10 | 338 | 1.84 |
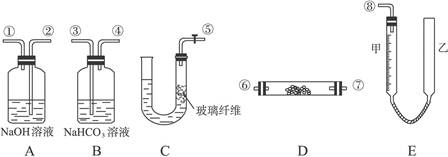
 ��װ�â����Ҳ�С�Թ��з��������������Ӧ���еIJ����ǣ�����С�Թܣ������Һ���� �����������ƣ���������
��װ�â����Ҳ�С�Թ��з��������������Ӧ���еIJ����ǣ�����С�Թܣ������Һ���� �����������ƣ��������� �������ã� ��������Ȼ�����_____�ڣ���ϡ����¡���������
�������ã� ��������Ȼ�����_____�ڣ���ϡ����¡����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ȡ25 g CuSO4��5H2O����1 Lˮ�� |
| B����CuSO4��5H2O����ȥ���ᾧˮ��ȡ16 g����ˮ�Ƴ�1 L��Һ |
| C����25 g CuSO4��5H2O����ˮ�Ƴ�1 L��Һ |
| D��ȡ12.5 g CuSO4��5H2O����500 mLˮ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com