
��Ӧʱ��t/h | 1 | 4 | 9 | 16 | 25 |
MgO���Y/nm NiO���Y/nm | 0.05a b | 0.20a 2b | 0.45a 3b | 0.80a 4b | 1.25a 5b |
ע��a��b��Ϊ���¶��йصij�����
����ջش�
��1����������������ʴ���ʿ����ý�������Ĥ��������������ʾ����������_________��
��2����������Ĥ��Ĥ��Y��ʱ��t�����ֵĹ�ϵ�ǣ�ֱ�ߡ������ߡ�������˫���ߵ����ͣ���MgO����Ĥ��Ĥ��Y��_________�ͣ�NiO����Ĥ��Ĥ��Y��___________ �͡�
��3��Mg��Ni�Ƚϣ����и����õ���������ʴ�Ե���___________ ��������___________ ��
 ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
������������ij�¶��£�����þ�����ֱ��������н���������Ӧʱ���ڽ�����������������Ĥ��ʵ���¼��
|
��Ӧʱ��t/h |
1 |
4 |
9 |
16 |
25 |
|
MgO���Y/nm NiO���Y/nm |
0.05a b |
0.20a 2b |
0.45a 3b |
0.80a 4b |
1.25a 5b |
ע��a��b��Ϊ���¶��йصij�����
����ջش�
(1)��������������ʴ���ʿ����ý�������Ĥ��������������ʾ����������___________��
(2)��������Ĥ��Ĥ��K��ʱ��t�����ֵĹ�ϵ��(ֱ�ߡ������ߡ�������˫���ߵ�����)��MgO����Ĥ��Ĥ��K��___________�ͣ�NiO����Ĥ��Ĥ��Y��_____________�͡�
(3)Mg��Ni�Ƚϣ����и����õ���������ʴ�Ե���_____________���������������������������������������������������������������������������������������������������������������������������������������� �������������������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022
|
��Ӧʱ��t/h |
1 |
4 |
9 |
16 |
25 |
|
MgO���Y/nm NiO���Y/nm |
0.05a b |
0.20a 2b |
0.45a 3b |
0.80a 4b |
1.25a 5b |
ע��a��b��Ϊ���¶��йصij�����
����ջش�
(1)��������������ʴ���ʿ����ý�������Ĥ��������������ʾ����������___________��
(2)��������Ĥ��Ĥ��K��ʱ��t�����ֵĹ�ϵ��(ֱ�ߡ������ߡ�������˫���ߵ�����)��MgO����Ĥ��Ĥ��K��___________�ͣ�NiO����Ĥ��Ĥ��Y��_____________�͡�
(3)Mg��
�鿴�𰸺ͽ���>>��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�߶��ڶ����¿���ѧ�Ծ� ���ͣ������
��9�֣��״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ����
�Ź�ҵ��һ��������з�Ӧ�ϳɼ״���CO(g)��2H2(g) CH3OH(g)����H
CH3OH(g)����H
�������������ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ����K����
|
250��: K1=__________ |
300��: K2=0.270 |
350��: K3=0.012 |
�ɱ��������жϦ�H 0����������������������� ��
��250�棬��2molCO��6molH2����2L���ܱ������У���ַ�Ӧ���ﵽƽ����:
COʣ��0.4mol����K1��
����֪�ڳ��³�ѹ�£���CH3OH��l��+O2��g��=CO��g��+2H2O��l������H=-442.8KJ/mol
��2CO(g)+O2(g)��2CO2(g)����H2 ����566.0kJ��mol
д���״�ȼ���ȵ��Ȼ�ѧ����ʽ____________________________________________��
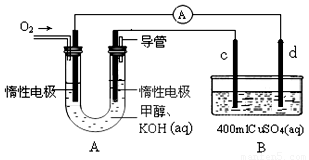
��ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���������ͼ(A)��ʾ��ȼ�ϵ��װ�á���
�ٸ�ȼ�ϵ�ظ����ĵ缫��ӦΪ��___________________________��
���øü״�ȼ�ϵ�ض�B�ؽ��е�⣬��֪c��d��������ͬ��ͭ�������2min��ȡ��c��d��ϴ������ɡ�������������Ϊ0.64g����ͨ������У���·��ͨ���ĵ���Ϊ_________mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��9�֣��״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ����
�Ź�ҵ��һ��������з�Ӧ�ϳɼ״���CO(g)��2H2(g)CH3OH(g)����H
�������������ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ����K����
| 250��: K1=__________ | 300��: K2=0.270 | 350��: K3=0.012 |
�ɱ��������жϦ�H 0�������������������������
��250�棬��2molCO��6molH2����2L���ܱ������У���ַ�Ӧ���ﵽƽ����:
COʣ��0.4mol����K1��
����֪�ڳ��³�ѹ�£���CH3OH��l��+O2��g��=CO��g��+2H2O��l������H=-442.8KJ/mol
��2CO(g)+O2(g)��2CO2(g)����H2����566.0kJ��mol
д���״�ȼ���ȵ��Ȼ�ѧ����ʽ____________________________________________��
��ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���������ͼ(A)��ʾ��ȼ�ϵ��װ�á���
�ٸ�ȼ�ϵ�ظ����ĵ缫��ӦΪ��___________________________��
���øü״�ȼ�ϵ�ض�B�ؽ��е�⣬��֪c��d��������ͬ��ͭ�������2min��ȡ��c��d��ϴ������ɡ�������������Ϊ0.64g����ͨ������У���·��ͨ���ĵ���Ϊ_________mol��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com