
 ij»ÆѧѧĻ°Š”×éµÄĶ¬Ń§ ŅĄ¾ŻŃõ»Æ»¹Ō·“Ó¦£ŗMnO4-+5Fe2++8H+ØTMn2++5Fe3++4H2O£¬æɲÉÓƵĪ¶ØµÄ·½·Ø²ā¶ØFeSO4µÄÖŹĮæ·ÖŹż£¬ŹµŃé²½ÖčČēĻĀ£ŗ
ij»ÆѧѧĻ°Š”×éµÄĶ¬Ń§ ŅĄ¾ŻŃõ»Æ»¹Ō·“Ó¦£ŗMnO4-+5Fe2++8H+ØTMn2++5Fe3++4H2O£¬æɲÉÓƵĪ¶ØµÄ·½·Ø²ā¶ØFeSO4µÄÖŹĮæ·ÖŹż£¬ŹµŃé²½ÖčČēĻĀ£ŗ·ÖĪö £Ø1£©KMnO4±ź×¼ČÜŅŗ¾ßÓŠĒæŃõ»ÆŠŌ£¬Ź¢×°µÄµĪ¶Ø¹ÜӦєȔĖįŹ½µĪ¶Ø¹Ü£»
£Ø2£©øßĆĢĖį¼Ų±¾Éķ³Ź×ĻÉ«£¬ĪŽŠčÖøŹ¾¼Į£»
£Ø3£©µĪ¶ØÖÕµćŹ±£¬ŃÕÉ«±ä³É×ĻŗģÉ«£¬²¢ĒŅ°ė·ÖÖÓÄŚ²»ĶŹÉ«¼“æÉ£»
£Ø4£©½«0.1mol•L-1µÄKMnO4ČÜŅŗ×°ČėµĪ¶Ø¹ÜÖŠ£¬µ÷½ŚŅŗĆęÖĮ8.00mL“¦£¬µĪ¶Ø“ż²āŅŗÖĮµĪ¶ØÖÕµćŹ±£¬µĪ¶Ø¹ÜµÄŅŗĆę¶ĮŹż18.00mL£¬ÓÉĢāŅāæÉÖŖ10.0ml“ż²āŅŗÓė10.0ml0.1mol•L-1µÄKMnO4ĶźČ«·“Ó¦£¬øł¾Ż5Fe2++MnO4-+8H+ØT5Fe3++Mn2++4H2O¼ĘĖć£»
£Ø5£©øł¾Żc£Ø“ż²ā£©V£Ø“ż²ā£©=c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©ÅŠ¶Ļ²»µ±²Ł×÷¶ŌĻą¹ŲĪļĄķĮæµÄÓ°Ļģ£®
½ā“š ½ā£ŗ£Ø1£©KMnO4±ź×¼ČÜŅŗ¾ßÓŠĒæŃõ»ÆŠŌ£¬ÄÜøÆŹ“Ļš½ŗ¹Ü£¬Ó¦Ź¢×°ŌŚĖįŹ½µĪ¶Ø¹ÜÖŠ£¬Ń”Ōń¼×£¬
¹Ź“š°øĪŖ£ŗ¼×£»
£Ø2£©øßĆĢĖį¼Ų±¾Éķ³Ź×ĻÉ«£¬µĪ¶ØĒ°ĪŽŠčµĪ¼ÓÖøŹ¾¼Į£¬
¹Ź“š°øĪŖ£ŗ·ń£»øßĆĢĖį¼Ų±¾Éķ³Ź×ĻÉ«£¬±»»¹ŌŗóČÜŅŗ»į±ä³ÉĪŽÉ«£»
£Ø3£©µĪ¶ØÖÕµćŹ±£¬ŃÕÉ«±ä³É×ĻŗģÉ«£¬²¢ĒŅ°ė·ÖÖÓÄŚ²»ĶŹÉ«£¬±¾ŹµŃé“ļµ½ÖÕµćµÄ±źÖ¾ŹĒ£ŗµĪČė×īŗóŅ»µĪKMnO4ČÜŅŗĒ”ŗĆÓÉ»ĘĀĢÉ«±äĒ³×ĻŗģÉ«£¬ĒŅ°ė·ÖÖÓÄŚ²»ĶŹÉ«£¬
¹Ź“š°øĪŖ£ŗµĪČė×īŗóŅ»µĪKMnO4ČÜŅŗĒ”ŗĆÓÉ»ĘĀĢÉ«±äĒ³×ĻŗģÉ«£¬ĒŅ°ė·ÖÖÓÄŚ²»ĶŹÉ«£»
£Ø4£©½«0.1mol•L-1µÄKMnO4ČÜŅŗ×°ČėµĪ¶Ø¹ÜÖŠ£¬µ÷½ŚŅŗĆęÖĮ8.00mL“¦£¬µĪ¶Ø“ż²āŅŗÖĮµĪ¶ØÖÕµćŹ±£¬µĪ¶Ø¹ÜµÄŅŗĆę¶ĮŹż18.00mL£¬ÓÉĢāŅāæÉÖŖ10.0ml“ż²āŅŗÓė10.0ml0.1mol•L-1µÄKMnO4ĶźČ«·“Ó¦£¬
5Fe2++MnO4-+8H+ØT5Fe3++Mn2++4H2O£¬
5 1
0.01L”ĮC 0.1mol•L-1”Į0.01L
ŌņC=0.5mol•L-1£¬Ōņ10.0ml“ż²āŅŗÖŠFeSO4µÄĪļÖŹµÄĮæĪŖ0.5mol•L-1”Į0.01L=0.005mol£¬FeSO4µÄÖŹĮæĪŖ0.005mol”Į152g/mol=0.76g£¬100mL“ż²āČÜŅŗÖŠFeSO4µÄÖŹĮæĪŖ7.6g£¬ĖłŅŌѳʷ֊FeSO4µÄÖŹĮæ·ÖŹżĪŖ$\frac{7.6g}{30.4g}$”Į110%=25%£¬
¹Ź“š°øĪŖ£ŗ25%£»
£Ø5£©¢ŁµĪ¶ØĒ°µĪ¶Ø¹Ü¼ā×ģ“¦ÓŠĘųÅŻĪ“Åųż£¬µĪ¶ØŗóĘųÅŻĻūŹ§£¬ĖłŠčv£Ø±ź×¼£©Ę«“ó£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©V£Ø±ź×¼£©}{V£Ø“ż²ā£©}$ÅŠ¶ĻæÉÖŖc£Ø“ż²ā£©Ę«øߣ¬
¹Ź“š°øĪŖ£ŗĘ«øߣ»
¢ŚČōµĪ¶ØĒ°ŃöŹÓµĪ¶Ø¹ÜæĢ¶Č¶ĮŹżŹ±£¬ĖłŠčv£Ø±ź×¼£©Ę«Š”£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©V£Ø±ź×¼£©}{V£Ø“ż²ā£©}$ÅŠ¶ĻæÉÖŖc£Ø“ż²ā£©Ę«µĶ£¬Ōņ»įŹ¹µĪ¶Ø½į¹ūĘ«µĶ£¬
¹Ź“š°øĪŖ£ŗĘ«µĶ£®
µćĘĄ ±¾ĢāÖ÷ŅŖæ¼²éĮĖ·“Ó¦ČČµÄ¼ĘĖć£¬ŃĪĄąĖ®½āµÄŌĖÓĆŅŌ¼°Ńõ»Æ»¹ŌµĪ¶Ø²ā¶ØĪļÖŹµÄŗ¬Į棬ĢāÄæÄѶČÖŠµČ£®


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
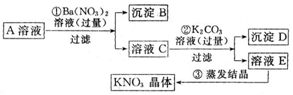
 ŅŃÖŖt”ꏱAgClµÄKsp=4”Į10-10£¬ŌŚt”ꏱ£¬Ag2CrO4ŌŚĖ®ÖŠµÄ³ĮµķČܽāĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾£®ĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ£Ø””””£©
ŅŃÖŖt”ꏱAgClµÄKsp=4”Į10-10£¬ŌŚt”ꏱ£¬Ag2CrO4ŌŚĖ®ÖŠµÄ³ĮµķČܽāĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾£®ĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ£Ø””””£©| A£® | ŌŚt”ꏱ£¬Ag2CrO4µÄKspĪŖ1”Į10-11 | |
| B£® | ŌŚ±„ŗĶČÜŅŗÖŠ¼ÓČėK2CrO4£Øs£©æÉŹ¹ČÜŅŗÓÉYµćµ½Zµć | |
| C£® | ŌŚt”ę£¬Ag2CrO4£Øs£©+2Cl-£Øaq£©?2AgCl£Øs£©+CrO${\;}_{4}^{2-}$£Øaq£©Ę½ŗā³£ŹżK=6.25”Į107 | |
| D£® | ŌŚt”ꏱ£¬ŅŌ0.001 mol•L-1 AgNO3ČÜŅŗµĪ¶Ø20 mL 0.001 mol•L-1 KClŗĶ0.001 mol•L-1µÄK2CrO4µÄ»ģŗĻČÜŅŗ£¬CrO${\;}_{4}^{2-}$ĻČ³Įµķ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ĪļÖŹ | Cu£ØOH£©2 | Fe£ØOH£©3 | CuCl | CuI |
| Ksp | 2.2”Į10-20 | 2.6”Į10-39 | 1.7”Į10-7 | 1.3”Į10-12 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ¶ŌŹå¶”»ł±½·Ó£Ø
¶ŌŹå¶”»ł±½·Ó£Ø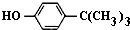 £©¹¤ŅµÓĆĶ¾¹ć·ŗ£¬æÉÓĆÓŚÉś²śÓĶČÜŠŌ·ÓČ©Ź÷Ö¬”¢ĪČ¶Ø¼ĮŗĶĻćĮĻµČ£®ŹµŃéŹŅŅŌ±½·Ó”¢Źå¶”»łĀČ[£ØCH3£©3CCl]µČĪŖŌĮĻÖʱø¶ŌŹå¶”»ł±½·Ó£®
£©¹¤ŅµÓĆĶ¾¹ć·ŗ£¬æÉÓĆÓŚÉś²śÓĶČÜŠŌ·ÓČ©Ź÷Ö¬”¢ĪČ¶Ø¼ĮŗĶĻćĮĻµČ£®ŹµŃéŹŅŅŌ±½·Ó”¢Źå¶”»łĀČ[£ØCH3£©3CCl]µČĪŖŌĮĻÖʱø¶ŌŹå¶”»ł±½·Ó£® £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
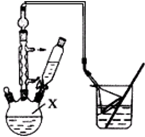
 1-äå±ūĶé³£ÓĆ×÷ÓŠ»ś·“Ó¦µÄČܼĮ£®ŹµŃéŹŅÖʱø1-äå±ūĶé£ØCH3CH2CH2Br£©µÄ·“Ó¦ŗĶÖ÷ŅŖŹµŃé×°ÖĆČēĻĀ£ŗ
1-äå±ūĶé³£ÓĆ×÷ÓŠ»ś·“Ó¦µÄČܼĮ£®ŹµŃéŹŅÖʱø1-äå±ūĶé£ØCH3CH2CH2Br£©µÄ·“Ó¦ŗĶÖ÷ŅŖŹµŃé×°ÖĆČēĻĀ£ŗ| Ļą¶Ō·Ö ×ÓÖŹĮæ | ĆÜ¶Č /g•mL-1 | ·Šµć/”ę | Ė®ÖŠ ČܽāŠŌ | |
| Õż±ū“¼ | 60 | 0.896 | 97.1 | ČÜ |
| Õż±ūĆŃ | 102 | 0.74 | 90 | ¼øŗõ²»ČÜ |
| 1-äå±ūĶé | 123 | 1.36 | 71 | ²»ČÜ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
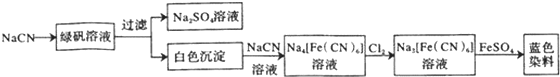
 £®
£®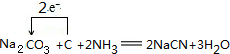 £®
£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ij»ÆѧѧĻ°Š”×éµÄŃŠ¾ææĪĢāŹĒ£ŗĢ½¾æ²ā¶Ø²ŻĖį¾§Ģå£ØH2C2O4•xH2O£©ÖŠxµÄÖµ£®øĆ×éĶ¬Ń§Ķعż²éŌÄ׏ĮĻ²éŃ°µĆ£¬²ŻĖįŅ×ČÜÓŚĖ®£¬Ė®ČÜŅŗæÉŅŌÓĆĖįŠŌKMnO4ČÜŅŗ½ųŠŠµĪ¶Ø£ŗ
ij»ÆѧѧĻ°Š”×éµÄŃŠ¾ææĪĢāŹĒ£ŗĢ½¾æ²ā¶Ø²ŻĖį¾§Ģå£ØH2C2O4•xH2O£©ÖŠxµÄÖµ£®øĆ×éĶ¬Ń§Ķعż²éŌÄ׏ĮĻ²éŃ°µĆ£¬²ŻĖįŅ×ČÜÓŚĖ®£¬Ė®ČÜŅŗæÉŅŌÓĆĖįŠŌKMnO4ČÜŅŗ½ųŠŠµĪ¶Ø£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com