
��֪ԭ���������ε�����5�ַǽ���Ԫ�أ�A��ԭ�Ӱ뾶������ԭ������С��BԪ����ɵĻ�����������࣬C���������������ڲ��������3��������Bͬ���ڣ�D��Bͬ���壬E��C����һ���ڣ�����ͬ����Ԫ���зǽ�������ǿ��Ԫ�ء�
�ش��������⣺
(1)��A��B��C��E����Ԫ���е�����Ԫ�ؿ��γɶ��ַ��ӣ����з��Ӣ�BC2����BA4����A2C2����BE4���������ڼ��Է��ӵ���________(�����)��
(2)C���⻯���������ͬ��Ԫ�ص��⻯��е㻹Ҫ�ߣ���ԭ����_______________��
(3)B��C��Ԫ�ض��ܺ�AԪ��������ֳ������ܼ��������ʽΪ________��________��DE4��ǰ���е��ܽ���________(����ڡ���С�ڡ�)�ں����е��ܽ��ԡ�
(4)BA4��BE4��DE4�ķе�Ӹߵ��͵�˳��Ϊ_________________(�ѧʽ)��
(5)A��C��E����Ԫ�ؿ��γɶ��ֺ����ᣬ��AEC��AEC2��AEC3��AEC4�ȣ������оٵ���������������ǿ������˳��Ϊ______________(�ѧʽ)��
 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ������ѧ�û�ѧ֪ʶ����Ҫ�����������йػ�ѧ�����ʾ��ȷ����
���õ���ʽ��ʾHCl���γɹ��̣� ��
��
��MgCl2 �ĵ���ʽ�� ��
��
��������Ϊ133��������Ϊ78���ԭ�ӣ� Cs
Cs
����ϩ�Ľṹ��ʽCH2CH2 ��
��п�̸ɵ���ڷŵ�ʱ�����ϵĵ缫��ӦʽΪ��Zn��Zn2++2e��
A���٢ڢۢܢ� B���ܢ� C���ۢ� D����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʯ�����ȼҵ�е�һ�ַ���������������±���ʾ��
| �ɷ� | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | CaS | ����������������� |
| �������� (%) | 65��66 | 3.5��5.0 | 1.5��3.5 | 0.2��0.8 | 0.2��1.1 | 1.0��1.8 | 23��26 |
�õ�ʯ����������ˮCaCl2��ij��������������¹������̣�
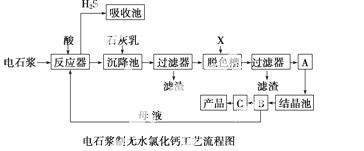
��֪�Ȼ��ƾ���Ļ�ѧʽ��CaCl2·6H2O��H2S��һ���������壬�Ҿ��л�ԭ�ԡ�
(1)��Ӧ���м������Ӧѡ�� ________��
(2)��ɫ����Ӧ���������X��______________���豸A��������______________���豸B������Ϊ ______________���豸C�������� ____________��
(3)Ϊ�����㻷��Ҫ���轫����H2Sͨ�����ճأ��������������ʺ���Ϊ���ռ�����________��
A��ˮ B��Ũ���� C��ʯ���� D������
(4)���豸B�в�����ĸҺ�������뷴Ӧ����Ŀ����__________________________
______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����
A���ϳɰ��ġ��������λ��������
B����Ƶ����Է�Һ�ü��кͺ�Ϳ����ŷ�
C����������Ĺ����У���Ϊ�������ϵ�����ú��������
D��ʹ��ú̿ת���Ĺܵ�ú����ֱ��ȼú�ɼ��ٻ�����Ⱦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ���������������ͼ���£�

��ش��������⣺
(1)�������������Ի�����Ϊԭ�ϣ������ڹ����������������Ϊԭ�ϣ�������________________________________________________________________________��
(2)������������Ӧ��ǰ�辻����ԭ����_________________________ ________
________
_____________________ ___________________________________________________��
___________________________________________________��
(3)�ڴ���Ӧ����ͨ��ʹ�ó�ѹ���ڴ�������SO2��ת����Ϊ90%�����Dz��ַ�����Ҳ�ȡ��ѹ��������ȡSO3����ȡ��ѹ��ʩ��Ŀ�ij��˼ӿ췴Ӧ�����⣬������____________________________���Ӷ��������Ч�ʡ�
(4)��ҵ�����г��ð�—�ᷨ����β�������Դﵽ������Ⱦ���������õ�Ŀ�ġ��û�ѧ����ʽ��ʾ�䷴Ӧԭ����____________________________________________________
________________________________________________________________________
________________________________________________________________________��
(5)�����Ṥҵ�⣬�������ҵ������������صĹ�ҵ������������ȷ����________��
A����ˮ���壺��ˮŨ��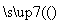
 ������
������
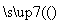
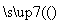 Һ��
Һ��
B����ˮ��þ����̲����
 ʯ��ˮ
ʯ��ˮ
 MgO
MgO þ
þ
C����ҵ��������� NO2
NO2 ���ᡪ��β������
���ᡪ��β������
D����ҵ�ϳɰ�����Ȼ�� ����
���� NH3��H2��N2
NH3��H2��N2 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ƶ�±����(NaX)���±����(SiX4)������������ȷ����
A��SiX4��ˮ��  B��SiX4�ǹ��ۻ�����
B��SiX4�ǹ��ۻ�����
C��NaX��ˮ�� D��NaX���۵�һ�����SiX4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ԫ��H��C��N��O��F������Ҫ�ķǽ���Ԫ��,Fe��Cu��Ӧ�÷dz��㷺�Ľ�����
(1)FeԪ�ػ�̬ԭ�ӵĺ�������Ų�ʽΪ����������
(2)C��HԪ���γɵĻ���������й���16������,�÷����ЦҼ���м��ĸ�����Ϊ����������
(3)C��N��O����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ(��Ԫ�ط��ű�ʾ)����������
(4)�ڲⶨHF����Է�������ʱ,ʵ����ֵһ���������ֵ,����Ҫԭ����
����
(5)C��N��Ԫ���γɵĻ�����C3N4�γɵ�ԭ�Ӿ���,�ṹ���ƽ��ʯ,����Ӳ�ȳ������ʯ,��ԭ����
����
(6)��ͼΪʯī�����ṹʾ��ͼ,�þ����к���Cԭ�ӵĸ���Ϊ����������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����a mol FeBr2����Һ�У�ͨ��x mol Cl2�����и���ΪͨCl2������ ����Һ�ڷ�����Ӧ�����ӷ���ʽ����������ȷ����
����Һ�ڷ�����Ӧ�����ӷ���ʽ����������ȷ����
A��x��0.4a��2Fe2-+Cl2��2Fe3++2Cl-
B��x��0.6a��2Br��+ Cl2��Br2+2Cl��
C��x=a��2Fe2��+2Br��+2Cl2��Br2+2Fe3��+4Cl��
D��x=1.5a��2Fe2��+4Br��+3Cl2��2Br2+2Fe3��+6Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڷ�ӦX��2Y===R��2M�У���֪R��M��Ħ������֮��Ϊ22��9����1.6 g X��Y��ȫ��Ӧ������4.4 g R�����ڴ˷�Ӧ��Y��M������֮��Ϊ(����)
A��16��9 B��23��9 C��32��9 D��46��9
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com