
A��B��C��X��Ϊ�����Ĵ��������֮��������ת����ϵ������Ʒ����ȥ����
�Իش�
��1����X��ǿ�����Ե��ʣ���A�������� ��
a. S b. N2 c.Na d.Mg e. Al
��2����X�ǽ������ʣ���C��ˮ��Һ�е���AgNO3��Һ������������ϡHNO3�İ�ɫ��������B�Ļ�ѧʽΪ ��C��Һ������ʱӦ��������X�������ǣ��ñ�Ҫ�����ֺ����ӷ���ʽ��ʾ�� ��
�����C��Һ�н���Ԫ�ؼ�̬�IJ��������� ��
��3����A��B��CΪ��ij����Ԫ�ص��������XΪǿ����ʣ�A��Һ��C��Һ��Ӧ����B����B�Ļ�ѧʽΪ ��X�Ļ�ѧʽ����Ϊ��д����ͬ�����ʣ� �� ��
��Ӧ�ٵ����ӷ���ʽΪ ��
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 Al��OH��3
Al��OH��3 Al3++3OH-
Al3++3OH- Al��OH��3
Al��OH��3 Al3++3OH-
Al3++3OH-

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| X | Y | Z |
| �� | �� | �� |
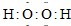
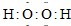
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��Kc | 9.94 | 9 | 1 |
| A | B | C | D | E | |
| n��CO2�� | 3 | 1 | 0 | 1 | 1 |
| n��H2�� | 2 | 1 | 0 | 1 | 2 |
| n��CO�� | 1 | 2 | 3 | 0.5 | 3 |
| n��H2O�� | 5 | 2 | 3 | 2 | 1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺
A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ������ش��������⣺
A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ������ش��������⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com