
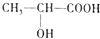
 �����������г������л���밴Ҫ������������⣻
�����������г������л���밴Ҫ������������⣻���� ��1�������ǵĽṹ��ʽΪCH2OH-CHOH-CHOH-CHOH-CHOH-CHO����������4��Cԭ����4����ͬԭ�ӻ�ԭ���������������Ƿ����е�̼��˫�������������ӳɣ�����CH2OH-CHOH-CHOH-CHOH-CHOH-CH2OH��
��2�������ڿ�����������������ܱ�����ΪCO2��H2O����������غ���д��ѧ����ʽ��
��3���л���A������[CH3CH��OH��COOH]�����Ժ��ֱ�����ϣ�ֻҪ����һ������ȫȼ�պ������ˮ������һ����Ӧ����A����Ԫ�ص�������������������Ԫ������������ȣ���������ʽΪCH2O����Ӧ�ĽṹΪ��Է���������С���л���Ϊ��ȩ�����������ʽ��ͬ����������Na2CO3��Ӧ�ų�CO2��˵�������Ȼ����ҷ�������һ��-CH3��Ӧ����������ΪHOCH2COOH��״�����������Ӧ�IJ��
��� �⣺��1�������ǵĽṹ��ʽΪCH2OH-CHOH-CHOH-CHOH-CHOH-CHO����������4��Cԭ����4����ͬԭ�ӻ�ԭ���������������Ƿ����е�̼��˫�������������ӳɣ�����CH2OH-CHOH-CHOH-CHOH-CHOH-CH2OH��������Ȼ��4��Cԭ����4����ͬԭ�ӻ�ԭ��������������4������̼ԭ�ӣ�
�ʴ�Ϊ��CH2OH-CHOH-CHOH-CHOH-CHOH-CHO��4��4��
��2�������ڿ�����������������ܱ�����ΪCO2��H2O����Ӧ�ķ���ʽΪC3H6O3+3O2$\stackrel{����}{��}$3CO2+3H2O���ʴ�Ϊ��C3H6O3+3O2$\stackrel{����}{��}$3CO2+3H2O��
��3���л���A������[CH3CH��OH��COOH]�����Ժ��ֱ�����ϣ�ֻҪ����һ������ȫȼ�պ������ˮ������һ����Ӧ����A����Ԫ�ص�������������������Ԫ������������ȣ�
��������ʽΪCH2O����Է���������С���л���A�Ľṹ��ʽΪHCHO��
��A���������ʽ��ͬ����������Na2CO3��Ӧ�ų�CO2��˵�������Ȼ����ҷ�������һ��-CH3����A�Ľṹ��ʽ������HOCH2COOCH3��
�ʴ�Ϊ��A����Ԫ�ص�������������������Ԫ������������ȣ�HCHO��HOCH2COOCH3��
���� ���⿼���л�����ƶϡ���������ͬ���칹�����д�ȣ�Ϊ��Ƶ���㣬������ѧ���ķ��������Ŀ��飬�Ѷ��еȣ����չ����ŵ�������ת���ǹؼ���ע�����֪ʶ�����գ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��

| A�� | ��������������̼ԭ��һ������ͬһƽ���� | |
| B�� | ��ϩ�Ʒӿɷ����ӳɡ�ȡ�����������Ӿۡ�������Ӧ | |
| C�� | �����ͼ�ϩ�ƷӶ����ڷ����廯���� | |
| D�� | ��1mol�������ͼ�ϩ�Ʒӷֱ����������3mol ��6mol Br2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
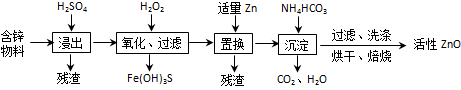
| A�� | ���������У�����ʱ�õ���60% H2SO4���ܶ���1.5g/cm3����������100 mL����H2SO4��Һ������Ҫ18.4mol•L-1��Ũ����ԼΪ49.9mL | |
| B�� | �����ɵij�������̬��ΪZna��OH��b ��CO3��c�ģ�a��b��c���������������ּ�ʽ̼��п�Ļ�����ֱ������Zn5��OH��6��CO3��2 ��Zn3��OH��6CO3 | |
| C�� | ������NH4HCO3�����ɵij�����Zn5��OH��6��CO3��2����÷�ӦΪ5ZnSO4+10NH4HCO3�TZn5��OH��6��CO3��2��+5��NH4��2SO4+8CO2��+2H2O | |
| D�� | ����������H2O2��ֻ��Fe��OH��3�������֣�����Һ��c��Fe3+���T2.6��10-15mol•L-1������Һ��c��Cu2+����2.2��10-4mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | ���� | ||
| C�� | 2-�����飨CH3��3CH | D�� | 2��2-�������飨CH3��4C |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
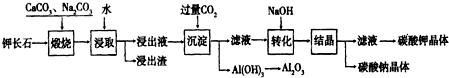
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
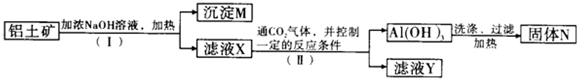
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
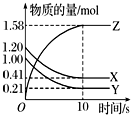 һ���¶��£���2L�ĺ����ܱ������У�X��Y��Z������������ʵ�����ʱ��仯��������ͼ��ʾ��
һ���¶��£���2L�ĺ����ܱ������У�X��Y��Z������������ʵ�����ʱ��仯��������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��t��ʱ��AgI��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����֪t��ʱAgBr��Ksp=5��10-13������˵������ȷ���ǣ�������
��t��ʱ��AgI��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����֪t��ʱAgBr��Ksp=5��10-13������˵������ȷ���ǣ�������| A�� | ��t��ʱ��AgI��Ksp=2.5��10-15 mol2•L-2 | |
| B�� | ͼ��b���е⻯���������� | |
| C�� | ��c����Һ�м�����������ˮ����ʹ��Һ��c�㵽a�� | |
| D�� | ��t��ʱ����ӦAgBr��s��+I-��aq��?AgI��s��+Br-��aq�� ��ƽ�ⳣ��K=200 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
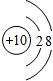 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com