
ijµŲĆŗķ·ŹÆ¾Ō¤“¦Ąķŗóŗ¬SiO2£Ø63%£©”¢Al2O3£Ø25%£©”¢Fe2O3£Ø5%£©¼°ÉŁĮæøĘĆ¾µÄ»ÆŗĻĪļµČ£¬Ņ»ÖÖ×ŪŗĻĄūÓĆ¹¤ŅÕÉč¼ĘČēĻĀ£ŗ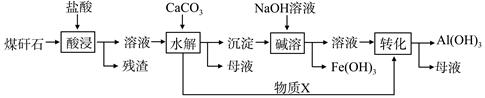
£Ø1£©”°Ėį½ž”±¹ż³ĢÖŠÖ÷ŅŖ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_____________”¢_________________”£
£Ø2£©”°Ėį½ž”±Ź±ĀĮ½ž³öĀŹµÄÓ°ĻģŅņĖŲæÉÄÜÓŠ_____________”¢___________”££ØŠ“³öĮ½øö£©
£Ø3£©ĪļÖŹXµÄ»ÆѧŹ½ĪŖ___________”£”°¼īČÜ”±Ź±·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ____________”£
£Ø4£©ŅŃÖŖFe3+æŖŹ¼³ĮµķŗĶ³ĮµķĶźČ«µÄpH·Ö±šĪŖ2.1ŗĶ3.2£¬Al3+æŖŹ¼³ĮµķŗĶ³ĮµķĶźČ«µÄpH·Ö±šĪŖ4.1ŗĶ5.4”£ĪŖĮĖ»ńµĆ²śĘ·Al£ØOH£© 3£¬“ÓĆŗķ·ŹÆµÄŃĪĖį½žČ”ŅŗæŖŹ¼£¬ČōÖ»ÓĆCaCO3Ņ»ÖÖŹŌ¼Į£¬ŗóŠų²Ł×÷¹ż³ĢŹĒ____________________”£
£Ø5£©ŅŌĆŗķ·ŹÆĪŖŌĮĻ»¹æÉŅŌæŖ·¢ĘäĖū²śĘ·£¬ĄżČēŌŚĆŗķ·ŹÆµÄŃĪĖį½žČ”Ņŗ³żĢśŗ󣬳£ĪĀĻĀĻņAlCl3ČÜŅŗÖŠ²»¶ĻĶØČėHClĘųĢ壬æÉĪö³ö“óĮæAlCl3”¤6H2O¾§Ģ壬½įŗĻ»ÆŃ§Ę½ŗāŅʶÆŌĄķ½āŹĶĪö³ö¾§ĢåµÄŌŅņ£ŗ_______________________”£
£Ø1£©Al2O3+6H+==2Al3++3H2O £» Fe2O3+6H+==2Fe3++3H2O £Ø4·Ö£¬Ćæøö2·Ö£©
£Ø2£©ŃĪĖįµÄÅØ¶Č”¢·“Ó¦ĪĀ¶Č”¢Ćŗķ·ŹÆæÅĮ£“óŠ””¢ŹĒ·ń³ä·Ö½Į°č”¢·“Ó¦Ź±¼ä£ØČĪŠ“Į½øö£©£Ø4·Ö£¬Ćæøö2·Ö£©
£Ø3£©CO2£» Al£ØOH£©3 +OH”Ŗ=AlO2”Ŗ+2H2O £Ø4·Ö£¬Ćæøö2·Ö£©
£Ø4£©¼ÓČėCaCO3µ÷½ŚpHµ½3.2£¬¹żĀĖ³żČ„Fe£ØOH£© 3ŗó£¬ŌŁ¼ÓČėCaCO3µ÷½ŚpHµ½5.4£¬¹żĀĖµĆµ½Al£ØOH£© 3 £Ø2·Ö£©
£Ø5£©AlCl3±„ŗĶČÜŅŗÖŠ“ęŌŚČܽāĘ½ŗā£ŗAlCl3”¤6H2O£Øs£© Al3+£Øaq£© +3Cl”Ŗ£Øaq£© +6H2O£Øl£©£¬ĶØČėHClĘųĢåŹ¹ČÜŅŗÖŠc£ØCl”Ŗ£©Ōö“ó£¬Ę½ŗāĻņĪö³ö¹ĢĢåµÄ·½ĻņŅĘ¶Æ“Ó¶ųĪö³öAlCl3¾§Ģ唣 £Ø2·Ö£©
Al3+£Øaq£© +3Cl”Ŗ£Øaq£© +6H2O£Øl£©£¬ĶØČėHClĘųĢåŹ¹ČÜŅŗÖŠc£ØCl”Ŗ£©Ōö“ó£¬Ę½ŗāĻņĪö³ö¹ĢĢåµÄ·½ĻņŅĘ¶Æ“Ó¶ųĪö³öAlCl3¾§Ģ唣 £Ø2·Ö£©
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©øł¾ŻĆŗķ·ŹÆ¾Ō¤“¦Ąķŗóŗ¬³É·ÖæÉÖŖ£¬ÄÜÓėHClÖ÷ŅŖ·“Ó¦µÄĪŖAl2O3”¢Fe2O3£¬Ęä·“Ó¦µÄĄė×Ó
·½³ĢŹ½ĪŖAl2O3+6H+==2Al3++3H2O”¢ Fe2O3+6H+==2Fe3++3H2O £»
£Ø2£©Ó°ĻģĖį½žŅņĖŲÓŠŃĪĖįµÄÅØ¶Č”¢·“Ó¦ĪĀ¶Č”¢±ķĆ껿£ØĆŗķ·ŹÆæÅĮ£“󊔣©”¢ŹĒ·ń³ä·Ö½Į°č”¢·“Ó¦Ź±¼äµČ£»
£Ø3£©Ėį½žŗóµÄČÜŅŗ³ŹĖįŠŌ£¬Ņ»·½ĆęŹĒŹ£
ÓąµÄHCl£¬ĮķŅ»·½ĆęŹĒAl3++3H2O Al£ØOH£©3+3H+”¢Fe3++3H2O
Al£ØOH£©3+3H+”¢Fe3++3H2O Fe£ØOH£©3+3H+µÄĖ®½ā³ŹĖįŠŌ£¬¼ÓČėCaCO3ŗóÓėĘäH+·“Ӧɜ³ÉCO2£»ŅņAl£ØOH£©3ŹĒĮ½ŠŌĒāŃõ»ÆĪļ£¬¼ČÄÜČÜŅŗĖį£¬ÓÖÄÜČÜÓŚĒæ¼ī£¬Ļņ³ĮµķÖŠ
Fe£ØOH£©3+3H+µÄĖ®½ā³ŹĖįŠŌ£¬¼ÓČėCaCO3ŗóÓėĘäH+·“Ӧɜ³ÉCO2£»ŅņAl£ØOH£©3ŹĒĮ½ŠŌĒāŃõ»ÆĪļ£¬¼ČÄÜČÜŅŗĖį£¬ÓÖÄÜČÜÓŚĒæ¼ī£¬Ļņ³ĮµķÖŠ
¼ÓČē¼īŹ±£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖAl£ØOH£©3 +OH”Ŗ=AlO2”Ŗ+2H2O£»
£Ø4£©øł¾ŻFe3+”¢Al3+³ĮµķµÄpHæÉÖŖ£¬Ź¹Fe3+³ĮµķŹ±£¬Al3+Ąė×Ó²»ÄܳĮµķ£¬¹ŹÓ¦½«pHµ÷ÖĮµ½3.2£¬ĪŖĮĖ»ńµĆ²śĘ·Al£ØOH£© 3£¬ŌņŠčŅŖAl3+Ąė×ÓĶźČ«³Įµķ£¬¹Ź½«pHµ÷ÖĮµ½5.4£¬ŌŚµŚ¶ž²½µ÷½ŚpHÖ®Ē°£¬Ó¦ÓĆ¹żĀĖµÄ·½·Ø½«Fe£ØOH£© 3³żČ„£»£Ø5£©AlCl3±„ŗĶČÜŅŗÖŠ“ęŌŚČܽāĘ½ŗā£¬ĶØČėHClĘųĢåČÜÓŚĖ®µēĄė³öCl”Ŗ£¬Ź¹ČÜŅŗÖŠc£ØCl”Ŗ£©Ōö“ó£¬Ę½ŗāĻņĪö³ö¹ĢĢåµÄ·½ĻņŅĘ¶Æ“Ó¶ųĪö³öAlCl3¾§Ģ唣
æ¼µć£ŗæ¼²é»Æѧ¹¤ŅÕĮ÷³ĢĶ¼”£Ö÷ŅŖæ¼²éÓŠĄė×Ó·½³ĢŹ½”¢Ó°Ļģ»Æѧ·“Ó¦µÄŅņĖŲ”¢ŌŖĖŲ¼°Ęä»ÆŗĻĪļµÄŠŌÖŹ”¢µ÷½ŚČÜŅŗµÄpHÖµ³żŌÓ”¢³ĮµķČܽāĘ½ŗā”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĪŁŹĒĪŅ¹ś·į²śŌŖĖŲ”£×ŌČ»½ēÖŠĪŁÖ÷ŅŖŅŌĪŁ(£«6)ĖįŃĪµÄŠĪŹ½“ęŌŚ”£ŗŚĪŁæóµÄÖ÷ŅŖ³É·ÖŹĒĢśŗĶĆĢµÄĪŁĖįŃĪ(FeWO4”¢MnWO4)”£ŗŚĪŁæó“«Ķ³Ņ±Į¶¹¤ŅÕĮ÷³ĢĶ¼ČēĻĀ£ŗ
(1)ŅŃÖŖÉĻŹö×Ŗ»ÆÖŠ£¬³ż×īŗóŅ»²½Ķā£¬WµÄ»ÆŗĻ¼ŪĪ“·¢Éś±ä»Æ£¬Ōņ²śĘ·CµÄ»ÆѧŹ½ĪŖ £»ČēŗĪ½«ĘäŅ±Į¶³Éµ„ÖŹ(ÓĆ»Æѧ·½³ĢŹ½±ķŹ¾)£ŗ ”£
øł¾Ż½šŹōµÄ»ī¶ÆŠŌ²»Ķ¬£¬½šŹōµÄŅ±Į¶·½·ØŅ»°ćÓŠ ČżÖÖ”£
(2)Š“³öµŚŅ»²½×Ŗ»ÆÖŠ”°Mn2£«”śMnO2”±µÄĄė×Ó·½³ĢŹ½ ”£
(3)ĪŅ¹śĪŁ»Æѧъ¾æµÄµģ»łČĖ¹ĖŅķ¶«ĻČÉś²ÉÓĆĮķĶāµÄ·“Ó¦ÖʵĆĮĖŅ»ÖÖĄ¶É«µÄ”¢·ĒÕū±ČµÄĪŁµÄŃõ»ÆĪļWO(3£x)”£ÕāÖÖĄ¶É«Ńõ»ÆĪŁ¾ßÓŠ±Č±ķĆę“ó”¢Ņ×»¹ŌµÄÓÅµć”£Ņ»°ćČĻĪŖ£¬Ą¶É«Ńõ»ÆĪŁµÄŃÕÉ«ŗĶ·ĒÕū±Č°µŹ¾ĮĖŌŚ»ÆŗĻĪļÖŠ“ęŌŚÕżĪå¼ŪŗĶÕżĮł¼ŪĮ½ÖÖ¼ŪĢ¬µÄĪŁ£¬ŅŃÖŖxµÄÖµĪŖ0.1£¬ŌņĄ¶É«Ńõ»ÆĪŁÖŠÕāĮ½ÖÖ¼ŪĢ¬µÄĪŁŌ×ÓŹżÖ®±ČĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÓŠŅ»ĪŽÉ«ČÜŅŗ£¬ĘäÖŠæÉÄÜŗ¬ÓŠFe3£«”¢Al3£«”¢Fe2£«”¢Mg2£«”¢Cu2£«”¢NH4+”¢K£«”¢CO32”Ŗ”¢SO42”ŖµČĄė×ÓÖŠµÄ¼øÖÖ£¬ĪŖ·ÖĪöĘä³É·Ö£¬Č”“ĖČÜŅŗ·Ö±š½ųŠŠĖÄøöŹµŃé£ŗ
¢ŁÕŗČ”ČÜŅŗ½ųŠŠŃęÉ«·“Ó¦(Ķø¹żĄ¶É«īܲ£Į§)ĻŌ×ĻÉ«£¬
¢Ś¼ÓČėŃĪĖįĖį»ÆµÄĀČ»Æ±µČÜŅŗ£¬³öĻÖ°×É«³Įµķ£¬
¢Ū¼ÓČė¹żŃõ»ÆÄĘ¹ĢĢ壬²śÉśĪŽÉ«ĪŽĪ¶µÄĘųĢåŗĶ°×É«³Įµķ£¬
¢ÜČ”100 mLøĆČÜŅŗÖšµĪ¼ÓČė¹żĮæµÄ5 mol”¤L£1ĒāŃõ»ÆÄĘČÜŅŗ£¬Éś³É°×É«³ĮµķÓė¼ÓČėĒāŃõ»ÆÄʵÄĮæČēĶ¼£ŗ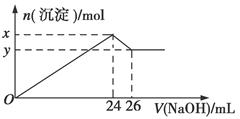
(1)ŌČÜŅŗÖŠŅ»¶Ø²»“ęŌŚµÄĄė×Ó£ŗ____________________________”£
(2)ĪŖĀś×ćČÜŅŗÖŠŅ»¶Ø“ęŌŚµÄĄė×ÓŅŖĒó£¬Ņ»°ćæÉČܽāĮ½ÖÖ³£¼ūµÄĪļÖŹ£¬Ęä»ÆѧŹ½ĪŖ________”¢________”£
x£y£½________mol”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijČÜŅŗæÉÄÜŗ¬ÓŠCl”„”¢SO42”„”¢CO32”„”¢NH4+”¢Fe3+”¢Fe2+”¢Al3+ŗĶNa+”£Ä³Ķ¬Ń§ĪŖĮĖČ·ČĻĘä³É·Ö£¬Č”²æ·ÖŹŌŅŗ£¬Éč¼Ę²¢Ķź³ÉĮĖČēĻĀŹµŃé£ŗ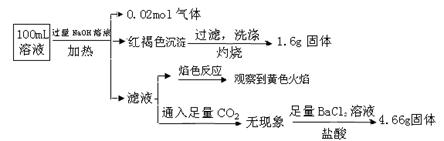
ÓÉ“ĖæÉÖŖŌČÜŅŗÖŠ
| A£®ŌČÜŅŗÖŠc£ØFe3+£©="0.2" mol”¤L-1 |
| B£®ČÜŅŗÖŠÖĮÉŁÓŠ4ÖÖĄė×Ó“ęŌŚ£¬ĘäÖŠCl”„Ņ»¶Ø“ęŌŚ£¬ĒŅc£ØCl”„£©”Ż0.2 mol”¤L-1 |
| C£®SO42”„”¢NH4+”¢Na+Ņ»¶Ø“ęŌŚ£¬CO32”„”¢Al3+Ņ»¶Ø²»“ęŌŚ |
| D£®ŅŖČ·¶ØŌČÜŅŗÖŠŹĒ·ńŗ¬ÓŠFe2+,Ęä²Ł×÷ĪŖ£ŗȔɣĮæŌČÜŅŗÓŚŹŌ¹ÜÖŠ,¼ÓČėŹŹĮæĀČĖ®£¬ĪŽĻÖĻó£¬ŌŁ¼ÓKSCNČÜŅŗ£¬ČÜŅŗ³ÉŃŖŗģÉ«£¬Ōņŗ¬ÓŠFe2+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ij³§·ĻĖ®ÖŠŗ¬5.00”Į10£3 mol”¤L£1µÄCr2O£¬Ę䶾ŠŌ½Ļ“ó”£Ä³ŃŠ¾æŠŌѧĻ°Š”×éĪŖĮĖ±ä·ĻĪŖ±¦£¬½«·ĻĖ®“¦ĄķµĆµ½“ÅŠŌ²ÄĮĻCr0.5Fe1.5FeO4(FeµÄ»ÆŗĻ¼ŪŅĄ“ĪĪŖ£«3”¢£«2)£¬Éč¼ĘĮĖČēĻĀŹµŃéĮ÷³Ģ£ŗ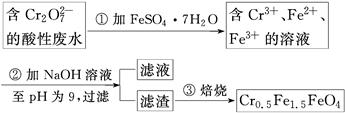
£Ø1£©µŚ¢Ł²½·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ________________________________________________”£
£Ø2£©µŚ¢Ś²½¹żĀĖµĆµ½µÄĀĖŌüÖŠÖ÷ŅŖ³É·Ö³żCr(OH)3Ķā£¬»¹ÓŠ__________”£
£Ø3£©ÓūŹ¹1 LøĆ·ĻĖ®ÖŠµÄCr2O72-ĶźČ«×Ŗ»ÆĪŖCr0.5Fe1.5FeO4”£ĄķĀŪÉĻŠčŅŖ¼ÓČė________g FeSO4”¤7H2O”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijæĪĶāŠ”×é¶ŌŅ»Š©½šŹōµ„ÖŹŗĶ»ÆŗĻĪļµÄŠŌÖŹ½ųŠŠĢ½¾æ”£
£Ø1£©ĻĀ±ķĪŖ”°ĀĮÓėĀČ»ÆĶČÜŅŗ·“Ó¦”±ŹµŃé±ØøęµÄŅ»²æ·Ö£ŗ
| ŹµŃé²½Öč | ŹµŃéĻÖĻó |
| ½«“ņÄ„¹żµÄĀĮʬ£Ø¹żĮ棩·ÅČėŅ»¶ØÅØ¶ČµÄCuCl2ČÜŅŗÖŠ | ²śÉśĘųÅŻ£¬Īö³öŹčĖɵÄŗģÉ«¹ĢĢ壬ČÜŅŗÖš½„±äĪŖĪŽÉ« |
| ·“Ó¦½įŹųŗó·ÖĄė³öČÜŅŗ±øÓĆ | |
| ŗģÉ«¹ĢĢåÓĆÕōĮóĖ®Ļ“µÓŗó£¬ÖĆÓŚ³±ŹŖæÕĘųÖŠ | Ņ»¶ĪŹ±¼äŗó¹ĢĢåÓÉŗģÉ«±äĪŖĀĢÉ«[ÉčĘäÖ÷ŅŖ³É·ÖĪŖCu2£ØOH£©2CO3] |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ķµ„ÖŹ¼°Ęä»ÆŗĻĪļŹĒÓ¦ÓĆ¼«Ęä¹ć·ŗµÄĪļÖŹ”£
(1)ĶŹĒĒāŗó½šŹō£¬²»ÄÜÓėŃĪĖį·¢ÉśÖĆ»»·“Ó¦£¬µ«½«µ„ÖŹĶÖĆÓŚÅØĒāµāĖįÖŠ£¬»įÓŠæÉČ¼ŠŌĘųĢå¼°°×É«³ĮµķÉś³É£¬ÓÖÖŖŃõ»ÆŠŌ£ŗCu2£«>I2£¬ŌņĶÓėĒāµāĖį·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ____________________________________________________”£
(2)ŅŃÖŖCu2OÄÜČÜÓŚ“×ĖįČÜŅŗ»ņŃĪĖįÖŠ£¬Ķ¬Ź±µĆµ½Ą¶É«ČÜŅŗŗĶŗģÉ«¹ĢĢ壬ŌņCu2OÓėĻ”ĮņĖį·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ____________________________________£»
Cu2OÓėĻ”ĻõĖį·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_____________________________£»
Ö»ÓĆĻ”ĮņĖįĄ“Č·¶ØijŗģÉ«¹ĢĢåŹĒ Cu2OÓėCu×é³ÉµÄ»ģŗĻĪļµÄ·½·Ø£ŗ³ĘČ”m gøĆŗģÉ«¹ĢĢåÖĆÓŚ×ćĮæĻ”ĮņĖįÖŠ£¬³ä·Ö·“Ó¦ŗó¹żĀĖ£¬Č»ŗó___________________”£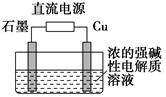
(3)Cu2OŹĒŅ»ÖÖ°ėµ¼Ģå²ÄĮĻ£¬»łÓŚĀĢÉ«»ÆѧĄķÄīÉč¼ĘµÄÖĘČ”Cu2OµÄµē½ā×°ÖĆČēĶ¼ĖłŹ¾£¬µē½ā×Ü·“Ó¦£ŗ2Cu£«H2Oµē½ā,Cu2O£«H2”ü£¬ŌņŹÆÄ«Ó¦ÓėµēŌ“µÄ________¼«ĻąĮ¬£¬Ķµē¼«ÉĻµÄµē¼«·“Ó¦Ź½ĪŖ________£»µē½ā¹ż³ĢÖŠ£¬Ņõ¼«ĒųÖÜĪ§ČÜŅŗpH________(Ģī”°±ä“ó”±”¢”°±äŠ””±»ņ”°²»±ä”±)”£
(4)ĻÖĻņCu”¢Cu2O”¢CuO×é³ÉµÄ»ģŗĻĪļÖŠ¼ÓČė1 L 0.6 mol/L HNO3Ē”ŗĆŹ¹»ģŗĻĪļČܽā£¬Ķ¬Ź±ŹÕ¼Æµ½2 240 mL NO(±ź×¼×“æö)”£Čō½«ÉĻŹö»ģŗĻĪļÓĆ×ćĮæµÄĒāĘų»¹Ō£¬ĖłµĆ¹ĢĢåµÄÖŹĮæĪŖ________£»Čō»ģŗĻĪļÖŠŗ¬ÓŠ0.1 mol Cu£¬½«øĆ»ģŗĻĪļÓėĻ”ĮņĖį³ä·Ö·“Ó¦£¬ÖĮÉŁĻūŗÄĮņĖįµÄĪļÖŹµÄĮæĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijĪŽÉ«“ż²āŅŗÖŠæÉÄÜŗ¬ÓŠAg+”¢Fe3+”¢K+”¢Ba2+”¢NH4+µČŃōĄė×Ó”£Ä³Ķ¬Ń§½ųŠŠČēĻĀŹµŃé£ŗ
I£®¼ÓČė¹żĮæµÄĻ”ŃĪĖį£¬ÓŠ°×É«³ĮµķÉś³É”£
II£®¹żĀĖ£¬Č”ÉŁŠķĀĖŅŗ£¬ĻņĘäÖŠ¼ÓČė¹żĮæµÄĻ”ĮņĖį£¬ÓÖÓŠ°×É«³ĮµķÉś³É”£
III£®ĮķȔɣĮæ²½ÖčIIÖŠµÄĀĖŅŗ£¬¼ÓČėNaOHČÜŅŗÖĮČÜŅŗ³Ź¼īŠŌ£¬¼ÓČČ£¬æɲśÉśŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶É«µÄĘųĢ唣
£Ø1£©“ż²āŅŗÖŠŅ»¶Øŗ¬ÓŠµÄĄė×ÓŹĒ__________£¬Ņ»¶Ø²»ŗ¬ÓŠµÄĄė×ÓŹĒ___________”£
£Ø2£©²½ÖčIIIÖŠ²śÉśĘųĢåµÄĄė×Ó·½³ĢŹ½ĪŖ__________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Š“³öĻĀĮŠ»Æѧ·½³ĢŹ½”¢Ąė×Ó·½³ĢŹ½»ņµēĄė·½³ĢŹ½£ŗ
£Ø1£©Ģ¼ĖįÄĘČÜŅŗŗĶ¹żĮæµÄ“×ĖįČÜŅŗ·“Ó¦£ØĄė×Ó·½³ĢŹ½£©
£Ø2£©Ć¾ŌŚ¶žŃõ»ÆĢ¼ĘųĢåÖŠČ¼ÉգػÆѧ·½³ĢŹ½£©
£Ø3£©“ĪĀČĖįµēĄė£ØµēĄė·½³ĢŹ½£©
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com