
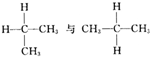
���� ��1��ͬ���칹����ָ����ʽ��ͬ�����ṹ��ͬ�Ļ����
ͬ����������ָ��ͬ��Ԫ����ɵIJ�ͬ���ʣ�
ͬλ������������ͬ������������ͬ��ԭ�ӣ�
ͬһ�����Ƿ��������ͬ���ṹ��ͬ�����ʣ�
ͬϵ��ָ�ṹ���ơ�ͨʽ��ͬ����������1���������ɸ�CH2ԭ���ţ�������ͬ�����ŵĻ����
��2�����������ƽ����ѧʽΪCxHy����Ӧǰ���ѹǿ���䣬����������ʵ������䣬��Ϸ�Ӧ�Ļ�ѧ����ʽ�жϣ�
��0.1molij����ȫȼ�����ɵIJ���ȫ��ͨ�������ļ�ʯ�ң�ʹ��ʯ������44.2g����H2O��CO2��������Ϊ44.2g����120�����ͬѹǿ�£����ɵ�CO2�������H2O�����������1.6������H2O�����ʵ���Ϊxmol����CO2�����ʵ���Ϊ1.6xmol������������18x+44��1.6x=44.2�����x=0.5����H2O�����ʵ���Ϊ0.5mol������CO2�����ʵ���Ϊ0.8mol������Ԫ���غ��ȷ�������ʻ�ѧʽ��
��� �⣺��1��A��11H��21H��������Ϊ1����������ͬ������Ԫ�صIJ�ͬԭ�ӣ���Ϊͬλ�أ�
B�����ʯ��ʯī��C60����CԪ����ɵIJ�ͬ���ʣ���Ϊͬ�������壻
C��������춡��ķ���ʽ��ͬ���ṹ��ͬ����Ϊͬ���칹�壻
D��C��CH3��3-CH3�� CH2-CH��CH3��-CH��CH3��-CH3�Ľṹ���ƣ�����������������̼ԭ������ͬ����Ϊͬϵ�
E��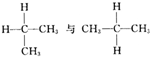 ���DZ��飬Ϊͬһ�����ʣ�
���DZ��飬Ϊͬһ�����ʣ�
�ʴ�Ϊ��C��A��B��E��D��
��2�����������ƽ����ѧʽΪCxHy��
���ݣ�CxHy+��x+$\frac{y}{4}$��O2=xCO2+$\frac{y}{2}$H2O
ȼ��ǰ�������ڣ��¶ȸ���100�棩ѹǿ���ֲ��䣬����������ʵ������䣬
���У�1+x+$\frac{y}{4}$=x+$\frac{y}{2}$����ã�y=4�������к��е�Hԭ��ƽ����Ϊ4��̼ԭ�Ӳ�ȷ�����������ƽ����ѧʽΪCxH4�����Ա�������Hԭ����ƽ��Ϊ4������������ΪAC�������ܵ�ΪBD��
�ʴ�Ϊ��BD��
��0.1molij����ȫȼ�����ɵIJ���ȫ��ͨ�������ļ�ʯ�ң�ʹ��ʯ������44.2g����H2O��CO2��������Ϊ44.2g����120�����ͬѹǿ�£����ɵ�CO2�������H2O�����������1.6������H2O�����ʵ���Ϊxmol����CO2�����ʵ���Ϊ1.6xmol������������18x+44��1.6x=44.2�����x=0.5����H2O�����ʵ���Ϊ0.5mol������CO2�����ʵ���Ϊ0.8mol������Ԫ���غ��֪�������ʻ�ѧʽΪ��C8H10��
�ʴ�Ϊ��C8H10��
���� ���⿼���Ϊ�ۺϣ��漰����ͬ�����жϡ��л������ʽȷ���ļ��㣬��Ŀ�Ѷ��еȣ���ȷͬϵ�ͬ���칹�塢ͬ�������塢ͬλ�صȸ���Ϊ���ؼ���ע�����������غ㶨����ȷ���л������ʽ�е�Ӧ�÷�����


 �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д� �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 4L | B�� | 8L | C�� | 10L | D�� | 12L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NO2ͨ��FeSO4��Һ�� | B�� | CO2ͨ��CaCl2��Һ�� | ||
| C�� | Cl2ͨ��Na2CO3��Һ�� | D�� | SO2ͨ���ữ��Ba��NO3��2��Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 25.5g | B�� | 39g | C�� | 51g | D�� | 106.5g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C2H6��C3H8û��ͬ���칹�壬CH2O2��C2H4O2����ͬϵ�� | |
| B�� | һ�������£��������������ۡ������ʡ���ϩ������ˮ����ˮ�ⷴӦ | |
| C�� | һ�������£���ȫȼ��14g������������Ϊa����ϩ����ȩ������壬������ˮ������Ϊ18��1-a��g | |
| D�� | ȫ�������ϣ�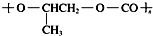 �����ɵ��廷�����飨 �����ɵ��廷�����飨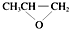 ����CO2�����Ƶ� ����CO2�����Ƶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѧ����Ϊ�Ҵ� | B�� | �ӷ� | ||
| C�� | �������л��ܼ� | D�� | ���ܱ����Ը���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
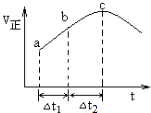
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com