
(10��)8��34gFeS04��7H20��Ʒ������ˮ���̵���������(��Ʒ�������¶ȱ仯������)����ͼ��ʾ��

��ش��������⣺
(1)��ȷ��78��ʱ��������M�Ļ�ѧʽ�� ��
(2)ȡ����380��ʱ���õ���ƷP����������������650�棬�õ�һ�ֹ�������Q��ͬʱ��������ɫ�������ɣ�д���÷�Ӧ�Ļ�ѧ����ʽ
(3)ij��ȤС������ͼ��ʾװ�����ʵ�飬��֤(2)�����ɵ���̬���ʣ����ⶨ�ѷֽ��P������(������װ���ڿ�����Ӱ��)��

���Լ�X�������� ��
�ڰ������������Ӹ�����������ĸ��ʾ�ӿڵ�����˳��c�� ��
�۳�ַ�Ӧ������װ��III��Բ����ƿ�ڻ����ⶨ�ѷֽ��P�����������������Ϊ����һ������Բ����ƿ����μ����Ȼ�����Һ��ֱ��������ȫ���ڶ��������˻����ڹ������Ͻ�����ϴ����ɲ���ȴ�����£����ء���������������ɡ���ȴ������ֱ���������γ��������������0��1gΪֹ�������յõ�����������ΪWg�����ѷֽ��P������ (�����ʽ) ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
. |
| v |
| t�� | 700 | 800 | 830 | 1000 | 1200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ľ�Ǵ���C5H12O5����һ����ζ�����������ǻ�Ϊͬ���칹�� | B��Ϊ��ֹ�±��ȸ�����֬��ʳƷ�������ʣ����ڰ�װ���з�����ʯ�� | C����Ч�ɷ�Ϊ̼��ƵIJ��Ƽ�������Ҫ���÷��ã�Ŀ�����ڴٽ��������� | D��BaSO4��BaCO3��Ksp����Ϊ1.08��10-10��8.1��10-9����BaSO4����ת��ΪBaCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ��ɫ��У������һ��������ѧ�Ծ� ���ͣ�ʵ����
(10��)8��34gFeS04��7H20��Ʒ������ˮ���̵���������(��Ʒ�������¶ȱ仯������)����ͼ��ʾ��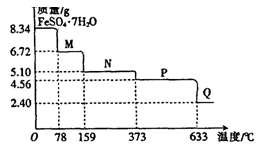
��ش��������⣺
(1)��ȷ��78��ʱ��������M�Ļ�ѧʽ�� ��
(2)ȡ����380��ʱ���õ���ƷP����������������650�棬�õ�һ�ֹ�������Q��ͬʱ��������ɫ�������ɣ�д���÷�Ӧ�Ļ�ѧ����ʽ
(3)ij��ȤС������ͼ��ʾװ�����ʵ�飬��֤(2)�����ɵ���̬���ʣ����ⶨ�ѷֽ��P������(������װ���ڿ�����Ӱ��)��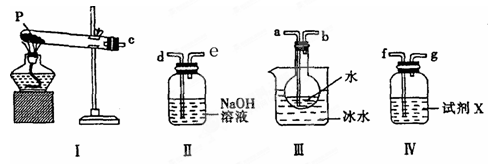
���Լ�X�������� ��
�ڰ������������Ӹ�����������ĸ��ʾ�ӿڵ�����˳��c�� ��
�۳�ַ�Ӧ������װ��III��Բ����ƿ�ڻ����ⶨ�ѷֽ��P�����������������Ϊ����һ������Բ����ƿ����μ����Ȼ�����Һ��ֱ��������ȫ���ڶ��������˻����ڹ������Ͻ�����ϴ����ɲ���ȴ�����£����ء���������������ɡ���ȴ������ֱ���������γ��������������0��1gΪֹ�������յõ�����������ΪWg�����ѷֽ��P������ (�����ʽ) ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)8��34gFeS04��7H20��Ʒ������ˮ���̵���������(��Ʒ�������¶ȱ仯������)����ͼ��ʾ��
��ش��������⣺
(1)��ȷ��78��ʱ��������M�Ļ�ѧʽ�� ��
(2)ȡ����380��ʱ���õ���ƷP����������������650�棬�õ�һ�ֹ�������Q��ͬʱ��������ɫ�������ɣ�д���÷�Ӧ�Ļ�ѧ����ʽ
(3)ij��ȤС������ͼ��ʾװ�����ʵ�飬��֤(2)�����ɵ���̬���ʣ����ⶨ�ѷֽ��P������(������װ���ڿ�����Ӱ��)��
���Լ�X�������� ��
�ڰ������������Ӹ�����������ĸ��ʾ�ӿڵ�����˳��c�� ��
�۳�ַ�Ӧ������װ��III��Բ����ƿ�ڻ����ⶨ�ѷֽ��P�����������������Ϊ����һ������Բ����ƿ����μ����Ȼ�����Һ��ֱ��������ȫ���ڶ��������˻����ڹ������Ͻ�����ϴ����ɲ���ȴ�����£����ء���������������ɡ���ȴ������ֱ���������γ��������������0��1gΪֹ�������յõ�����������ΪWg�����ѷֽ��P������ (�����ʽ) ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com