
ΓΨΧβΡΩΓΩΆ≠ΉΣ¬·―ΧΜ“÷ς“ΣΚ§”–ZnΘέΜΙ”–…ΌΝΩΒΡFe(+2Φέ)ΓΔPbΓΔCuΓΔAsΒ»‘ΣΥΊΘίΒΡΝρΥα―ΈΚΆ―θΜ·ΈοΘ§…ΌΝΩΈΣ…ιΥα―ΈΓΘ÷Τ±Η÷Ί“ΣΜ·ΙΛ‘≠ΝœΜν–‘―θΜ·–ΩΒΡΙΛ“’Νς≥Χ»γΆΦΥυ ΨΓΘ«κΜΊ¥π“‘œ¬Έ ΧβΘΚ
ΦΚ÷ΣΘΚΜν–‘ΧΩΨΜΜ·÷ς“Σ «≥ΐ»Ξ”–Μζ‘”÷ ΓΘ
(1)–¥≥ω¬»Μ·οßΒΡΒγΉ” Ϋ___Θ§ΓΑΨΜΜ·Γ±Ιΐ≥Χ τ”Ύ___(ΧνΓΑΈοάμΓ±ΓΔΓΑΜ·―ßΓ±)±δΜ·ΓΘ
(2)‘ΎΖ¥”ΠΈ¬Ε»ΈΣ50ΓφΘ§Ζ¥”Π ±ΦδΈΣ1h ±Θ§≤βΕ®Ης‘ΣΥΊΒΡΫΰ≥ω¬ ”ꬻ̷οß»ή“Κ≈®Ε»ΒΡΙΊœΒ»γΆΦΘ§‘ρ¬»Μ·οß “ΥΒΡ≈®Ε»ΈΣ___molΓΛL-1ΓΘ»τΫΰ≥ω“Κ÷––Ω‘ΣΥΊ“‘[Zn(NH3)4]2+–Έ Ϋ¥φ‘ΎΘ§‘ρΫΰ»Γ ±ZnOΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ___ΓΘ
(3)ΒΈΦ”KMnO4»ή“Κ”–MnO2…ζ≥…Θ§ΡΩΒΡ «≥ΐ___‘ΣΥΊΘ§≥ΐ‘”3 «÷ΟΜΜ≥ΐ‘”Ιΐ≥ΧΘ§‘ρ ‘ΦΝa «___Θ§ΓΑ¬Υ‘ϋΔσΓ±ΒΡ÷ς“Σ≥…Ζ÷ΈΣ___(ΧνΜ·―ß Ϋ)ΓΘ
(4)–¥≥ωΓΑ≥Ν–ΩΓ± ±ΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ___Θ§¥ΥΙΐ≥Χ÷–Ω…“‘―≠ΜΖάϊ”ΟΒΡΗ±≤ζΤΖ «___ΓΘ
(5)»ΓmgΜν–‘―θΜ·–Ω―υΤΖ≈δ≥…¥ΐ≤β“ΚΘ§Φ”»κ÷Η ΨΦΝ3ΓΔ4ΒΈΘ§‘ΌΦ”»κ ΝΩΝυ―«ΦΉΜυΥΡΑΖΘ§”ΟamolL-1EDTA±ξΉΦ“ΚΫχ––ΒΈΕ®Θ§œϊΚΡ±ξΉΦ“ΚVmLΓΘΦΚ÷ΣΘΚ”κ1.0mLEDTA±ξΉΦ“Κ[c(EDTA)=1.000mo1L-1]œύΒ±ΒΡ“‘ΩΥ±μ ΨΒΡ―θΜ·–Ω÷ ΝΩΈΣ0.08139Θ§‘ρ―υΤΖ÷–―θΜ·–ΩΒΡ÷ ΝΩΖ÷ ΐΈΣ___(”Ο¥ζ ΐ Ϋ±μ Ψ)ΓΘ
ΓΨ¥πΑΗΓΩ![]() Έοάμ 4 ZnO+2NH
Έοάμ 4 ZnO+2NH![]() +2NH3H2O=[Zn(NH3)4]2++3H2O Fe Zn Cu(Zn) 2Zn2++4HCO
+2NH3H2O=[Zn(NH3)4]2++3H2O Fe Zn Cu(Zn) 2Zn2++4HCO![]() =Zn2(OH)2CO3Γΐ+3CO2Γϋ+H2O NH4Cl
=Zn2(OH)2CO3Γΐ+3CO2Γϋ+H2O NH4Cl ![]() ΓΝ100%Μρ
ΓΝ100%Μρ![]() %
%
ΓΨΫβΈωΓΩ
Ήœ»άϊ”Ο¬»Μ·οßΚΆΑ±Υ°Ϋΰ»ΓΆ≠ΉΣ¬·―ΧΜ“Θ§Pb‘ΣΥΊΉΣΜ·ΈΣPb(OH)Cl≥ΝΒμ≥ΐ»ΞΘ§ZnΓΔFeΓΔCuΓΔAsΒ»‘ΣΥΊΫχ»κ»ή“ΚΘ§Φ”»κ¬»Μ·ΧζAs‘ΣΥΊΉΣΜ·ΈΣFeAsO4≥ΝΒμ≥ΐ»ΞΘ§Φ”»κΝρΥαΥαΜ·ΒΡΗΏΟΧΥαΦΊΘ§Fe‘ΣΥΊ»Ϊ≤Ω±Μ―θΜ·≥…Fe3+Θ§ΒςΫΎpH ΙFe‘ΣΥΊΉΣΜ·ΈΣ≥ΝΒμ≥ΐ»ΞΘΜ¥Υ ±ΜΙ”–Cu‘ΣΥΊΜα”ΑœλΚσ–χΦθ…ΌΧΦΥα–ΩΒΡ¥ΩΕ»Θ§Υυ“‘ ‘ΦΝa”Π≥ΐ»ΞΆ≠‘ΣΥΊΘ§ΈΣΝΥ≤Μ“ΐ»κ–¬ΒΡ‘”÷ Θ§Ω…“‘Φ”»κΙΐΝΩΒΡZnΒΞ÷ ÷ΟΜΜ≥ωΆ≠Θ§¥”ΕχΫΪΤδ≥ΐ»ΞΘΜ‘ΌΦ”»κΜν–‘ΧΩΨΜΜ·ΘΜ÷°ΚσΦ”»κΧΦΥα«βοßΫΪZn‘ΣΥΊΉΣΜ·ΈΣΦν ΫΧΦΥα–ΩΘ§λ―…’ΚσΒΟΒΫ―θΜ·–ΩΓΘ
(1)¬»Μ·οß”…οßΗυΚΆ¬»άκΉ”ΙΙ≥…Θ§ΒγΉ” ΫΈΣ![]() ΘΜΜν–‘ΧΩΈϋΗΫ”–Μζ‘”÷ ¥οΒΫΨΜΜ·ΒΡΡΩΒΡΘ§ΟΜ”––¬Έο÷ …ζ≥…Θ§ τ”ΎΈοάμ±δΜ·ΘΜ
ΘΜΜν–‘ΧΩΈϋΗΫ”–Μζ‘”÷ ¥οΒΫΨΜΜ·ΒΡΡΩΒΡΘ§ΟΜ”––¬Έο÷ …ζ≥…Θ§ τ”ΎΈοάμ±δΜ·ΘΜ
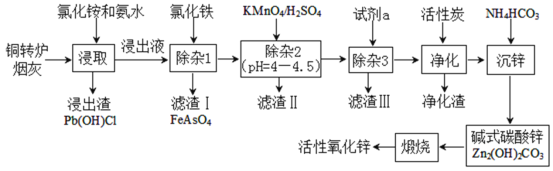
(2)Ϋΰ»ΓΙΐ≥Χ÷––η“Σ ΙZn‘ΣΥΊΨΓΩ…ΡήΕύΒΡΫχ»κ»ή“ΚΘ§Εχ‘”÷ άκΉ”“ΣΨΓΝΩ…ΌΒΡΫχ»κ»ή“ΚΘ§Ά§ ±ΈΣΝΥΦ”ΩλΖ¥”ΠΥΌ¬ Θ§–η“Σ¬»Μ·οßΒΡ≈®Ε»ΨΓΝΩ¥σ“Μ–©Θ§Ιέ≤λΧβΆΦΘ§Ω…÷ΣΒ±¬»Μ·οßΒΡ≈®Ε»ΈΣ4mol/L ±Θ§“ΜΖΫΟφ–Ω‘ΣΥΊΒΡΫΰ≥ω¬ “―Ψ≠Ϋ”Ϋϋ100%Θ§¬»Μ·οßΒΡ≈®Ε»“≤Ϋœ¥σΘ§Νμ“ΜΖΫΟφ»τ≈®Ε»‘ΌΗΏΘ§«Π‘ΣΥΊΫΪΫχ»κ»ή“ΚΘ§Υυ“‘Ήν “ΥΒΡ≈®Ε»ΈΣ4mol/LΘΜ
Ζ¥”ΠΈο”–ZnOΓΔ¬»Μ·οßΓΔ“ΜΥ°ΚœΑ±Θ§≤ζΈο”–[Zn(NH3)4]2+Θ§ΗυΨί‘ΣΥΊ ΊΚψΩ…ΒΟάκΉ”ΖΫ≥Χ ΫΈΣZnO+2NH![]() +2NH3H2O=[Zn(NH3)4]2++3H2OΘΜ
+2NH3H2O=[Zn(NH3)4]2++3H2OΘΜ
(3)ΒΈΦ”KMnO4»ή“ΚΩ…ΫΪFe2+―θΜ·ΈΣFe3+Θ§‘ΌΒςΫΎpH÷Β≥ΐ»ΞΘ§Υυ“‘ΡΩΒΡ «≥ΐ»ΞFe‘ΣΥΊΘΜΗυΨίΖ÷ΈωΩ…÷Σ ‘ΦΝaΈΣZnΘ§¬Υ‘ϋΔσ÷ς“Σ”–÷ΟΜΜ≥ωά¥ΒΡCuΚΆΈ¥Ζ¥”ΠΒΡZnΘΜ
(4)»ή“Κ÷–”–¥σΝΩ–ΩάκΉ”Θ§Φ”»κΧΦΥα«βοßΚσ≤ζ…ζZn2(OH)2CO3Θ§ΥΒΟςΧΦΥα«βΗυΒγάκ≥ωΒΡΧΦΥαΗυΚΆ–ΩάκΉ”ΓΔ«β―θΗυΫαΚœ…ζ≥…Φν ΫΧΦΥα–Ω≥ΝΒμΘ§¥ΌΫχΧΦΥα«βΗυΒΡΒγάκΘ§Βγάκ≥ωΒΡ«βάκΉ””÷ΚΆΧΦΥα«βΗυΫαΚœ…ζ≥…Εΰ―θΜ·ΧΦΚΆΥ°Θ§Υυ“‘άκΉ”ΖΫ≥Χ ΫΈΣ2Zn2++4HCO![]() =Zn2(OH)2CO3Γΐ+3CO2Γϋ+H2OΘΜΗυΨί«Α–ρ≤Ϋ÷ηΧμΦ”ΒΡΈο÷ Θ§“‘ΦΑ¥ΥΙΐ≥ΧΖΔ…ζΒΡΖ¥”ΠΩ…÷ΣΘ§¥Υ ±»ή“Κ÷–ΒΡ÷ς“Σ≥…Ζ÷ΈΣ¬»Μ·οßΚΆ…ΌΝΩΝρΥαΑ¥Θ§¬»Μ·οßΩ…“‘―≠ΜΖ Ι”ΟΘΜ
=Zn2(OH)2CO3Γΐ+3CO2Γϋ+H2OΘΜΗυΨί«Α–ρ≤Ϋ÷ηΧμΦ”ΒΡΈο÷ Θ§“‘ΦΑ¥ΥΙΐ≥ΧΖΔ…ζΒΡΖ¥”ΠΩ…÷ΣΘ§¥Υ ±»ή“Κ÷–ΒΡ÷ς“Σ≥…Ζ÷ΈΣ¬»Μ·οßΚΆ…ΌΝΩΝρΥαΑ¥Θ§¬»Μ·οßΩ…“‘―≠ΜΖ Ι”ΟΘΜ
(5)”ΟamolL-1EDTA±ξΉΦ“ΚΫχ––ΒΈΕ®Θ§œϊΚΡ±ξΉΦ“ΚVmLΘ§”κ1.0mLEDTA±ξΉΦ“Κ[c(EDTA)=1.000mo1L-1]œύΒ±ΒΡ“‘ΩΥ±μ ΨΒΡ―θΜ·–Ω÷ ΝΩΈΣ0.08139Θ§Υυ“‘m(ZnO)= aVΓΝ0.08139gΘ§÷ ΝΩΖ÷ ΐΈΣ![]() ΓΝ100%Μρ
ΓΝ100%Μρ![]() %ΓΘ
%ΓΘ


 ÷–ΩΦάϊΫΘ÷–ΩΦ ‘ΨμΜψ±ύœΒΝ–¥πΑΗ
÷–ΩΦάϊΫΘ÷–ΩΦ ‘ΨμΜψ±ύœΒΝ–¥πΑΗ ΫΧ”ΐ άΦ“Ή¥‘ΣΨμœΒΝ–¥πΑΗ
ΫΧ”ΐ άΦ“Ή¥‘ΣΨμœΒΝ–¥πΑΗ ΜΤΗ‘ΩΈΧΟΉς“Β±ΨœΒΝ–¥πΑΗ
ΜΤΗ‘ΩΈΧΟΉς“Β±ΨœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΜ·―ßΙΛ“ΒΈΣ“Ώ«ιΖάΩΊΧαΙ©ΝΥ«Ω”–ΝΠΒΡΈο÷ ÷ß≥≈ΓΘ¬»ΒΡ–μΕύΜ·ΚœΈοΦ» «÷Ί“ΣΜ·ΙΛ‘≠ΝœΘ§”÷ «ΗΏ–ßΓΔΙψΤΉΒΡΟπΨζœϊΕΨΦΝΓΘΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)¬»Τχ «÷Τ±ΗœΒΝ–Κ§¬»Μ·ΚœΈοΒΡ÷ς“Σ‘≠ΝœΘ§Ω…≤…”Ο»γΆΦ(a)Υυ ΨΒΡΉΑ÷Οά¥÷Τ»ΓΓΘΉΑ÷Ο÷–ΒΡάκΉ”ΡΛ÷Μ‘ –μ______άκΉ”Ά®ΙΐΘ§¬»ΤχΒΡ“ί≥ωΩΎ «_______(Χν±ξΚ≈)ΓΘ
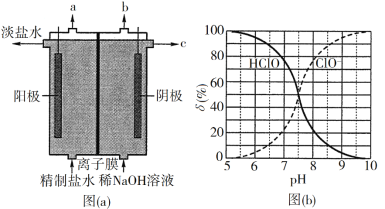
(2)¥Έ¬»ΥαΈΣ“Μ‘Σ»θΥαΘ§ΨΏ”–Τ·ΑΉΚΆ…±ΨζΉς”ΟΘ§ΤδΒγάκΤΫΚβΧεœΒ÷–Ης≥…Ζ÷ΒΡΉι≥…Ζ÷ ΐΠΡ[ΠΡ(X)=![]() Θ§XΈΣHClOΜρClO]”κpHΒΡΙΊœΒ»γΆΦ(b)Υυ ΨΓΘHClOΒΡΒγάκ≥Θ ΐKa÷ΒΈΣ______ΓΘ
Θ§XΈΣHClOΜρClO]”κpHΒΡΙΊœΒ»γΆΦ(b)Υυ ΨΓΘHClOΒΡΒγάκ≥Θ ΐKa÷ΒΈΣ______ΓΘ
(3)Cl2OΈΣΒ≠ΉΊΜΤ…ΪΤχΧεΘ§ «¥Έ¬»ΥαΒΡΥατϊΘ§Ω…”…–¬÷ΤΒΡHgOΚΆCl2Ζ¥”Πά¥÷Τ±ΗΘ§ΗΟΖ¥”ΠΈΣΤγΜ·Ζ¥”Π(―θΜ·ΦΝΚΆΜΙ‘≠ΦΝΈΣΆ§“Μ÷÷Έο÷ ΒΡΖ¥”Π)ΓΘ…œ ω÷Τ±ΗCl2OΒΡΜ·―ßΖΫ≥Χ ΫΈΣ______ΓΘ
(4)ClO2≥ΘΈ¬œ¬ΈΣΜΤ…ΪΤχΧεΘ§“Ή»ή”ΎΥ°Θ§ΤδΥ°»ή“Κ «“Μ÷÷ΙψΤΉ…±ΨζΦΝΓΘ“Μ÷÷”––ß≥…Ζ÷ΈΣNaClO2ΓΔNaHSO4ΓΔNaHCO3ΒΡΓΑΕΰ―θΜ·¬»≈ίΧΎΤ§Γ±Θ§ΡήΩλΥΌ»ή”ΎΥ°Θ§“γ≥ω¥σΝΩΤχ≈ίΘ§ΒΟΒΫClO2»ή“ΚΓΘ…œ ωΙΐ≥Χ÷–Θ§…ζ≥…ClO2ΒΡΖ¥”Π τ”ΎΤγΜ·Ζ¥”ΠΘ§ΟΩ…ζ≥…1 mol ClO2œϊΚΡNaClO2ΒΡΝΩΈΣ_____molΘΜ≤ζ…ζΓΑΤχ≈ίΓ±ΒΡΜ·―ßΖΫ≥Χ ΫΈΣ____________ΓΘ
(5)ΓΑ84œϊΕΨ“ΚΓ±ΒΡ”––ß≥…Ζ÷ΈΣNaClOΘ§≤ΜΩ…”κΥα–‘«εΫύΦΝΜλ”ΟΒΡ‘≠“ρ «______(”ΟάκΉ”ΖΫ≥Χ Ϋ±μ Ψ)ΓΘΙΛ“Β…œ «ΫΪ¬»ΤχΆ®»κΒΫ30%ΒΡNaOH»ή“Κ÷–ά¥÷Τ±ΗNaClO»ή“ΚΘ§»τNaClO»ή“Κ÷–NaOHΒΡ÷ ΝΩΖ÷ ΐΈΣ1%Θ§‘ρ…ζ≤ζ1000 kgΗΟ»ή“Κ–ηœϊΚΡ¬»ΤχΒΡ÷ ΝΩΈΣ____kg(±ΘΝτ’ϊ ΐ)ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΡœΨ©«ύΑ¬ΜαΦΣœιΈοΈΣΓΑ≥i≥iΓ±Θ§“Μ÷÷ΓΑ≥i≥iΓ±ΒΡΆβ”Ο≤ΡΝœ «¥Ω―ρΟΪœΏΘ§ΡΎ≥δΈοΈΣΒ”¬ΎΘ®ΫαΙΙΦρ ΫΈΣ![]() Θ§œ¬Ν–”–ΙΊΥΒΖ®≤Μ’ΐ»ΖΒΡ «Θ® Θ©
Θ§œ¬Ν–”–ΙΊΥΒΖ®≤Μ’ΐ»ΖΒΡ «Θ® Θ©
A.Ω…”ΟΉΤ…’ΒΡΖΫΖ®«χ±π―ρΟΪΚΆΒ”¬Ύ
B.Κœ≥…Β”¬ΎΒΡΒΞΧε÷°“Μ «HOCH2CH2OH
C.Β”¬Ύ τ”ΎΧλ»ΜΗΏΖ÷Ή”Μ·ΚœΈο
D.―ρΟΪΚΆΒ”¬ΎΥυΚ§‘ΣΥΊ≤ΜΆξ»ΪœύΆ§
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΑ±≈πΆι(NH3BH3)Κ§«βΝΩΗΏΓΔ»»Έ»Ε®–‘ΚΟΘ§ «“Μ÷÷ΨΏ”–«±ΝΠΒΡΙΧΧε¥Δ«β≤ΡΝœΓΘΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)HΓΔBΓΔN÷–Θ§‘≠Ή”ΑκΨΕΉν¥σΒΡ «______ΓΘΗυΨίΕ‘Ϋ«œΏΙφ‘ρΘ§BΒΡ“Μ–©Μ·―ß–‘÷ ”κ‘ΣΥΊ______ΒΡœύΥΤΓΘ
(2)NH3BH3Ζ÷Ή”÷–Θ§NΓΣBΜ·―ßΦϋ≥ΤΈΣ____ΦϋΘ§ΤδΒγΉ”Ε‘”…____ΧαΙ©ΓΘΑ±≈πΆι‘Ύ¥ΏΜ·ΦΝΉς”Οœ¬Υ°Ϋβ ΆΖ≈«βΤχΘΚ3NH3BH3+6H2O=3NH3+![]() +9H2Θ§
+9H2Θ§![]() ΒΡΫαΙΙ»γΆΦΥυ ΨΘΚ
ΒΡΫαΙΙ»γΆΦΥυ ΨΘΚ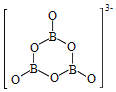 ΘΜ‘ΎΗΟΖ¥”Π÷–Θ§B‘≠Ή”ΒΡ‘”Μ·ΙλΒάάύ–Ά”…______±δΈΣ______ΓΘ
ΘΜ‘ΎΗΟΖ¥”Π÷–Θ§B‘≠Ή”ΒΡ‘”Μ·ΙλΒάάύ–Ά”…______±δΈΣ______ΓΘ
(3)NH3BH3Ζ÷Ή”÷–Θ§”κN‘≠Ή”œύΝ§ΒΡH≥ ’ΐΒγ–‘(HΠΡ+)Θ§”κB‘≠Ή”œύΝ§ΒΡH≥ ΗΚΒγ–‘(HΠΡ-)Θ§ΒγΗΚ–‘¥σ–ΓΥ≥–ρ «__________ΓΘ”κNH3BH3‘≠Ή”Ήή ΐœύΒ»ΒΡΒ»ΒγΉ”Χε «_________(–¥Ζ÷Ή” Ϋ)Θ§Τδ»έΒψ±»NH3BH3____________(ΧνΓΑΗΏΓ±ΜρΓΑΒΆΓ±)Θ§‘≠“ρ «‘ΎNH3BH3Ζ÷Ή”÷°ΦδΘ§¥φ‘Ύ____________________Θ§“≤≥ΤΓΑΥΪ«βΦϋΓ±ΓΘ
(4)―–ΨΩΖΔœ÷Θ§ΚΛ≈πΆι‘ΎΒΆΈ¬ΗΏ―ΙΧθΦΰœ¬ΈΣ’ΐΫΜΨßœΒΫαΙΙΘ§ΨßΑϊ≤Έ ΐΖ÷±πΈΣa pmΓΔb pmΓΔc pmΘ§ΠΝ=Π¬=ΠΟ=90ΓψΓΘΑ±≈πΆιΒΡ2ΓΝ2ΓΝ2≥§ΨßΑϊΫαΙΙ»γΆΦΥυ ΨΓΘ
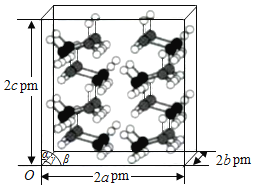
Α±≈πΆιΨßΧεΒΡΟήΕ»Π―=___________gΓΛcm3(Ν–≥ωΦΤΥψ ΫΘ§…ηNAΈΣΑΔΖϋΦ”Β¬¬ό≥Θ ΐΒΡ÷Β)ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΡήΥΒΟςSiO2ΈΣΥα–‘―θΜ·ΈοΒΡ ¬ ΒΈΣΘ® Θ©
A.SiO2”κH2SO4ΓΔHNO3≤ΜΖ¥”ΠB.SiO2ΘΪ4HF=SiF4ΓϋΘΪ2H2O
C.SiO2ΘΪ2KOH=K2SiO3ΘΪH2OD.SiO2ΘΪ2C![]() SiΘΪ2COΓϋ
SiΘΪ2COΓϋ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ¥”ΙηΒΡ―θΜ·ΈοΩ…“‘÷Τ»ΓΙηΒΞ÷ Θ§÷ς“ΣΜ·―ßΖ¥”Π»γœ¬ΘΚ¥÷ΙηΒΡ÷Τ»ΓΘΚ
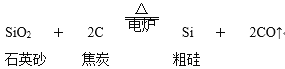
”…¥÷Ιη÷Τ¥ΩΙη(≥Θ”ΟΖΫΖ®)ΘΚSi(¥÷)ΘΪ2Cl2![]() SiCl4 SiCl4ΘΪ2H2
SiCl4 SiCl4ΘΪ2H2![]() Si(¥Ω)ΘΪ4HCl
Si(¥Ω)ΘΪ4HCl
ΗυΨί“‘…œΖ¥”ΠΘ§ΜΊ¥πœ¬Ν–Έ ΧβΓΘ
Θ®1Θ©‘Ύ÷Τ»Γ¥÷ΙηΒΡΖ¥”Π÷–Θ§ΫΙΧΩΒΡΉς”Ο « ≤Ο¥___ΘΩ
Θ®2Θ©‘Ύ”…¥÷Ιη÷Τ¥ΩΙηΒΡΖ¥”Π÷–Θ§¬»Τχ(Cl2)”κSiΒΡΖ¥”Π τ”Ύ ≤Ο¥άύ–ΆΒΡΖ¥”Π___ΘΩSiCl4”κH2ΒΡΖ¥”Π τ”Ύ ≤Ο¥άύ–ΆΒΡΖ¥”Π___ΘΩH2ΒΡΉς”Ο « ≤Ο¥___ΘΩ
Θ®3Θ©‘ΎΑκΒΦΧεΙΛ“Β÷–”–’β―υ“ΜΨδ––ΜΑΘΚΓΑ¥”…≥Χ≤ΒΫ”ΟΜßΓ±Θ§Ρψ «»γΚΈάμΫβΒΡ___ΘΩ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
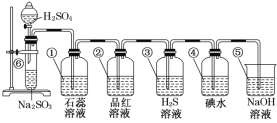
ΓΨΧβΡΩΓΩ»γΆΦΥυ Ψ «÷Τ»ΓSO2≤Δ―ι÷ΛSO2Ρ≥–©–‘÷ ΒΡΉΑ÷ΟΆΦΓΘ
‘ΜΊ¥πΘΚ
Θ®1Θ©Δό÷–ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ___ΓΘ
Θ®2Θ©ΔΌ÷–ΒΡ Β―ιœ÷œσΈΣ___Θ§¥Υ Β―ι÷ΛΟςSO2 «___―θΜ·ΈοΓΘ
Θ®3Θ©ΔΎ÷–ΒΡΤΖΚλ»ή“Κ___Θ§÷ΛΟςSO2”–___ΓΘ
Θ®4Θ©Δέ÷–ΒΡH2S»ή“Κ____Θ§÷ΛΟςSO2”–___ΓΘ
Θ®5Θ©Δή÷–ΒΡΒβΥ°___Θ§÷ΛΟςSO2”–____ΓΘ
Θ®6Θ©Δί÷–NaOH»ή“ΚΒΡΉς”Ο «___Θ§”–ΙΊΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ____ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΡ≥–Υ»Λ–ΓΉι…ηΦΤ≥ω»γΆΦΥυ ΨΉΑ÷Οά¥ΗΡΫχΫΧ≤Ρ÷–ΓΑΆ≠”κœθΥαΖ¥”ΠΓ± Β―ιΘ§“‘»Ζ±ΘΜ·―ß Β―ιΒΡ¬Χ…ΪΜ·ΓΘ
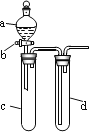
(1) Β―ι«ΑΘ§ΙΊ±’Μν»ϊbΘ§ ‘Ιήd÷–Φ”Υ°÷ΝΫΰΟΜ≥ΛΒΦΙήΩΎΘ§»ϊΫτ ‘ΙήcΚΆdΒΡΫΚ»ϊΘ§Φ”»»cΓΘΤδΡΩΒΡ «_____________________________________________________________________ΓΘ
(2)‘Ύd÷–Φ” ΝΩNaOH»ή“ΚΘ§c÷–Ζ≈“Μ–ΓΩιΆ≠Τ§Θ§”…Ζ÷“Κ¬©ΕΖaœρc÷–Φ”»κ2 mLΒΡ≈®œθΥαΓΘΖ¥”Π“ΜΕΈ ±ΦδΚσΘ§‘Ό”…aœρc÷–Φ”2 mL’τΝσΥ°Θ§c÷–ΒΡ Β―ιœ÷œσ”– ≤Ο¥±δΜ·___________ΓΘ
ΖΫΑΗ | ΦΉ | ““ | ±ϊ |
Ζ¥”ΠΈο | CuΓΔ≈®HNO3 | CuΓΔœΓHNO3 | CuΓΔO2ΓΔœΓHNO3 |
(3)»γ±μΥυ Ψ «÷Τ»ΓœθΥαΆ≠ΒΡ»ΐ÷÷ΖΫΑΗΘ§ΡήΧεœ÷¬Χ…ΪΜ·―ßάμΡνΒΡΉνΦ―ΖΫΑΗ «__________Θ§άμ”… «________________________________________________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΡ«Ω…ΕΓ «“Μ÷÷“©ΈοΘ§ΗΟ“©Έο ”Ο”Ύ¥ΧΦΛ–‘Η…Ω»≤Γ»ΥΖΰ”ΟΘ§Έό≥…ώΪ–‘Θ§Μ·ΚœΈοH «÷Τ±ΗΗΟ“©ΈοΒΡ÷Ί“Σ÷–ΦδΧεΘ§Κœ≥…¬ΖœΏ»γœ¬ΘΚ
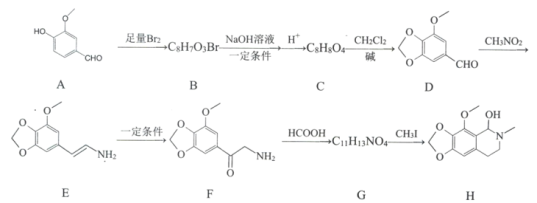
“―÷ΣΘΚΔΌ
ΔΎRNH2 ![]() RNHCH3
RNHCH3
Θ®1Θ©Μ·ΚœΈοBΒΡΫαΙΙΦρ ΫΘΚ________ΓΘ
Θ®2Θ©Ζ¥”ΠBΓζCΒΡΒΎ“Μ≤ΫΖ¥”Πάύ–ΆΘΚ____________ΓΘ
Θ®3Θ©œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «ΘΚ___________ΓΘ
A Έο÷ DΡή”κFeCl3ΖΔ…ζœ‘…ΪΖ¥”Π B Έο÷ FΨΏ”–Φν–‘
C Έο÷ GΡήΚΆ“χΑ±»ή“ΚΖΔ…ζΖ¥”Π D Έο÷ HΒΡΖ÷Ή” Ϋ «C12H15NO4
Θ®4Θ©–¥≥ωCΓζDΒΡΜ·―ßΖΫ≥Χ ΫΘΚ____________ΓΘ
Θ®5Θ©«κ–¥≥ωΜ·ΚœΈοH¬ζΉψœ¬Ν–ΧθΦΰΒΡΥυ”–Ά§Ζ÷“λΙΙΧεΒΡΫαηέΦρ ΫΘΚ_______________ΓΘ
ΔΌΖ÷Ή”÷–Κ§±ΫΜΖΘ§ΈόΤδΥϊΜΖΉ¥ΫαΙΙ
ΔΎΖ÷Ή”÷–Κ§”–NO2«“÷±Ϋ”Ν§‘Ύ±ΫΜΖ…œ
ΔέΖ÷Ή”÷–÷Μ”–3÷÷≤ΜΆ§Μ·―ßΜΖΨ≥ΒΡ«β
Θ®6Θ©“―÷ΣCH2=CHCH3![]() CH2CHCH2ClΘ§«κ“‘
CH2CHCH2ClΘ§«κ“‘![]() ΓΔCH3CHClCH3ΈΣ‘≠ΝœΚœ≥…Μ·ΚœΈο
ΓΔCH3CHClCH3ΈΣ‘≠ΝœΚœ≥…Μ·ΚœΈο![]() Θ§–¥≥ω÷Τ±ΗΒΡΚœ≥…¬ΖœΏΝς≥ΧΆΦΘ®ΈόΜζ ‘ΦΝ»Έ―ΓΘ©______________ΓΘ
Θ§–¥≥ω÷Τ±ΗΒΡΚœ≥…¬ΖœΏΝς≥ΧΆΦΘ®ΈόΜζ ‘ΦΝ»Έ―ΓΘ©______________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com