
���ú�̼������ϳ�ȼ���ǽ����ԴΣ������Ҫ��������֪CO(g)��2H2(g)CH3OH(g)��Ӧ�����е������仯�����ͼ��ʾ�����ߢ�����ߢ�ֱ��ʾ��ʹ�ô�����ʹ�ô�������������������ж���ȷ����(����)
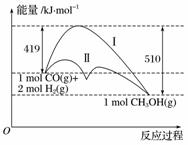
A���÷�Ӧ�Ħ�H��91 kJ·mol��1
B������������÷�Ӧ�Ħ�H��С
C����Ӧ��������������������������
D������÷�Ӧ����Һ̬CH3OH����H����
 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��̬��ԭ�ӵĵ����Ų�ʽΪ
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ж�������ȷ�Ļ����̡�������Ļ�������
(1)S(s)��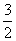 O2(g)===SO3(g)����H����315 kJ·mol��1(ȼ����)��(��H����ֵ��ȷ)(����)
O2(g)===SO3(g)����H����315 kJ·mol��1(ȼ����)��(��H����ֵ��ȷ)(����)
(2)NaOH(aq)��HCl(aq)===NaCl(aq)��H2O(l)
��H����57.3 kJ·mol��1(�к���)��(��H����ֵ��ȷ)(����)
(3)��֪H��(aq)��OH��(aq)===H2O(l)����H����57.3 kJ·mol��1����H2SO4��Ba(OH)2��Ӧ�ķ�Ӧ�Ȧ�H��2��(��57.3) kJ·mol��1(����)
(4)ȼ�ϵ���н��״�����ת��Ϊ�������Ȼ�ѧ����ʽ��CH3OH(g)��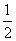 O2(g)===CO2(g)��2H2(g)
O2(g)===CO2(g)��2H2(g)
��H����192.9 kJ·mol��1����CH3OH(g)��ȼ����Ϊ192.9 kJ·mol��1(����)
(5)H2(g)��ȼ������285.8 kJ·mol��1����2H2O(g)===2H2(g)��O2(g)����H��571.6 kJ·mol��1(����)
(6)�����ǵ�ȼ������2 800 kJ·mol��1����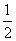 C6H12O6(s)��3O2(g)===3CO2(g)��3H2O(l)����H����1 400 kJ·mol��1(����)
C6H12O6(s)��3O2(g)===3CO2(g)��3H2O(l)����H����1 400 kJ·mol��1(����)
(7)��֪101 kPaʱ��2C(s)��O2(g)===2CO(g)
��H����221 kJ·mol��1����÷�Ӧ�ķ�Ӧ��Ϊ221 kJ·mol��1(����)
(8)��֪ϡ��Һ�У�H��(aq)��OH��(aq)===H2O(l)
��H����57.3 kJ·mol��1����ϡ������ϡ����������Һ��Ӧ����1 molˮʱ�ų�57.3 kJ������(����)
(9)��֪HCl��NaOH��Ӧ���к��Ȧ�H����57.3 kJ·mol��1����98%��Ũ������ϡ����������Һ��Ӧ����1 molˮ���к���Ϊ��57.3 kJ·mol��1(����)
(10)CO(g)��ȼ������283.0 kJ·mol��1����2CO2(g)===2CO(g)��O2(g)��Ӧ�Ħ�H��2��283.0 kJ·mol��1(����)
(11)������ȼ����Ϊ285.5 kJ·mol��1������ˮ���Ȼ�ѧ����ʽΪ2H2O(l)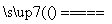 2H2(g)��O2(g)����H��285.5 kJ·mol��1(����)
2H2(g)��O2(g)����H��285.5 kJ·mol��1(����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��P4(g)��6Cl2(g)===4PCl3(g)��
��H��a kJ·mol��1��
P4(g)��10Cl2(g)===4PCl5(g)����H��b kJ·mol��1��
P4������������ṹ��PCl5��P—Cl���ļ���Ϊc kJ·mol��1��PCl3��P—Cl���ļ���Ϊ1.2c kJ·mol��1��
����������ȷ����(����)
A��P—P���ļ��ܴ���P—Cl���ļ���
B������Cl2(g)��PCl3(g)===PCl5(s)�ķ�Ӧ�Ȧ�H
C��Cl—Cl���ļ���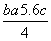 kJ·mol��1
kJ·mol��1
D��P—P���ļ���Ϊ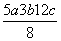 kJ·mol��1
kJ·mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����(����)
A��S(g)��O2(g)===SO2(g)����H1��S(s)��O2===SO2(g)����H2����H1����H2
B��C(ʯī��s)===C(���ʯ��s)����H��1.9 kJ·mol��1������ʯī��ȡ���ʯ�ķ�Ӧ�����ȷ�Ӧ�����ʯ��ʯī�ȶ�
C��NaOH(aq)��HCl(aq)===NaCl(aq)��H2O(l)����H����57.4 kJ·mol��1����20 g NaOH��ϡ��Һ��ϡ������ȫ��Ӧ���ų�������Ϊ28.7 kJ
D��2C(s)��O2(g)===2CO(g)����H����221 kJ·mol��1����̼��ȼ���ȵ���110.5 kJ·mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ԫ�����ڱ���λ�ÿ���̼���Ϊ��A��Ԫ�أ���Ȼ������̼�Ķ��ֵ��ʴ��ڣ���Ȼ�����й�ĵ�����Ϊʲô��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڹ赥�ʼ��仯�����˵����ȷ����(����)
��ˮ������һ�ֿ��コ���Ȳ���ȼ��Ҳ���ܸ�ʴ
��ˮ�ࡢ������ɳ�Ӷ��ǹ�������Ʒ
�۸ߴ��ȵĹ赥�ʹ㷺�����������ά
���մ�������Ӧ�ú���Ĺ����β���
A���٢� B���ڢ� C���٢� D���ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������CO2����ͨ��ˮ����(Na2SiO3��Һ)�У�Ȼ��������ɣ����ڸ����³�����գ����õ��Ĺ���������(����)
A��Na2SiO3 B��Na2CO3��Na2SiO3
C��Na2CO3��SiO2 D��SiO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����NaCl��NaBr��NaI����ѡ�õ��Լ���(����)
�ٵ�ˮ��������Һ������ˮ��CCl4������ˮ��������ϡ���ᡢAgNO3��Һ������ˮ��������FeCl3��Һ��CCl4
A���٢ڢ� B���ڢۢ� C���ڢܢ� D���ܢݢ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com