

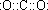 ���ʴ�Ϊ��NH3
���ʴ�Ϊ��NH3 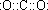 ��
��
| ||
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����0�棬ѹǿΪ101��105Paʱ��22.4L Cl2��HCl�Ļ�������к��е���ԭ������Ϊ3NA |
| B��0.5molI-������ʱʧȥ�ĵ�����Ϊ0.5NA |
| C��0.1L3 mol?L-1��NH4NO3��Һ�к��е�NH4+��ĿΪ0.3��6.02��1023 |
| D�����³�ѹ�£�48gO3������ԭ����Ϊ3NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������Һ�м����������ˮ�ټӵ��ۣ���������ɫ��˵��û��I- |
| B����FeI2��Һ�еμ�������ˮʱ�����ӷ�ӦʽΪ��2I+Cl2=I2+2Cl |
| C����ij��Һ�м���ϡ���ᣮ������������ʹ����ʯ��ˮ����ǣ�˵������Һ����CO32-��HCO3- |
| D������Һ���ȼ���ϡHNO3���ټ�BaCl2��Һ���а�ɫ�������ɣ�˵����SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
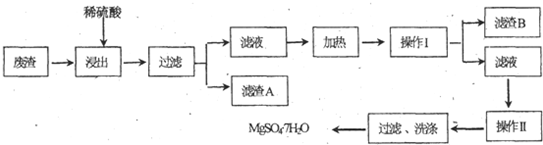

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢� | B���ڢ� | C���ۢ� | D���٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | CH3COOH | HCN | H2CO3 |
| Ka | 1.8��10-5 | 4.9��10-10 | K1��4.3��10-7 K2��5.6��10-11 |
| A��������ʵ���Ũ����ҺpH��ϵ��pH ��NaCN����pH ��Na2CO3����pH ��CH3COONa�� |
| B��������ʵ���Ũ�ȵ�HCN��NaCN�����Һ�У�c ��HCN��+c��H+��=c ��OH-��+c ��CN-�� |
| C��a mol/LHCN��b mol/LNaOH��Һ�������Ϻ����õ���Һ�У���c��Na+����c ��CN-������aһ��С��b |
| D����Na2CO3���뵽HCN��Һ��ʱ�������·�Ӧ��Na2CO3+HCN=NaCN+NaHCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��FeCl3��Һ�У�Al3+��K+��MnO4-��SO42- |
| B�����ȳʻ�ɫ����Һ�У�K+��Br-��S2-��ClO- |
| C���������۲�����������Һ�У�Na+��NH4+��NO3-��Cl- |
| D��Kw/c��OH-��=0.1mol/L����Һ�У�Na+��K+��AlO2-��CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����25mLˮ��������ʢ��5.4mLŨ�������Ͳ�У����ò��������Ͻ��� |
| B����10g98%��Ũ�������ձ�������ע��ʢ��25mLˮ���ձ��У����ò��������Ͻ��� |
| C��������������ȷ����ȡ25mLˮʱ���Ӷ�����������ϡ���������ʵ���������С��28% |
| D�����Ƹ���Һʱ������Ũ����մ������Ӧ����������������Һ�к� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com