
| ĪĀ¶Č£ØK£© | Ź§ÖŲ£Ø%£© | |
| µŚŅ»½×¶Ī | 323”«523 | 40.54 |
| µŚ¶ž½×¶Ī | 553”«687 | 48.65 |
| µŚČż½×¶Ī | 1 043ŅŌÉĻ | 84.68 |
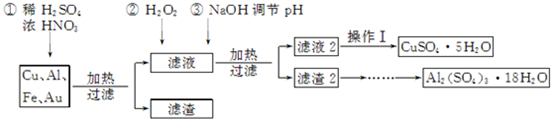
·ÖĪö Cu”¢Fe”¢Al”¢AuµÄ»ģŗĻĪļÖŠ¼ÓČėĮņĖįŗĶÅØĻõĖįµÄ»ģŗĻĪļČܽā£¬æÉŅŌµĆµ½ĀĖŅŗ1ĪŖĢśŃĪ”¢ĀĮŃĪ”¢ĶŃĪČÜŅŗ£¬ĀĖŌü1ĪŖAu£¬ĻņĀĖŅŗ1ÖŠ¼ÓČėĖ«ŃõĖ®ŗĶĒāŃõ»ÆÄĘ£¬¼ÓH2O2µÄ×÷ÓĆŹĒ°ŃFe2+Ńõ»ÆĪŖFe3+£¬½įŗĻ³ĮµķµÄpHæÉÖŖ£¬µ÷½ŚpHĪŖ£¼4£¬¹żĀĖ·ÖĄė³öĒāŃõ»ÆĢś£¬ŌŁµ÷½ŚpHĪŖ5×óÓŅ£¬¹żĀĖµĆĀĖŅŗ2ĪŖĮņĖįĶČÜŅŗ£¬ĀĖŌü2ĪŖAl£ØOH£©3£¬¼ÓĮņĖįČܽāŗó½į¾§µĆµ½¾§Ģ壬
£Ø1£©ŌŚ½šŹō»ģŗĻĪļÓėĖįµÄ·“Ó¦ÖŠ£¬ĻõĖį³äµ±Ńõ»Æ¼Į£¬øł¾ŻÉĻĆęµÄ·ÖĪöæÉÖŖĀĖŌü1µÄ³É·Ż£»
£Ø2£©²Ł×÷IĪŖÕō·¢”¢ÅØĖõ”¢ĄäČ“½į¾§£»¹żĀĖŠčŅŖĀ©¶·£»
£Ø3£©Ąė×Ó²»ČÜÓŚŅŅ“¼£»
£Ø4£©µŚ¢Ś²½¼ÓH2O2ŹĒĪŖĮĖ½«Fe2+Ńõ»ÆĪŖFe3+£»
£Ø5£©ÉčĖłČ”ĮņĖįĀĮ¾§ĢåĪŖ1 mol£¬ŌņĮņĖįĀĮ¾§ĢåµÄÖŹĮæĪŖ666 g
ŌŚµŚŅ»½×¶Ī£ŗ
¼ÓČČŗóŹ§Č„½į¾§Ė®µÄÖŹĮæŹĒ£ŗm£ØH2O£©=40.54%”Į666 g=270 g£¬
Ź§Č„½į¾§Ė®µÄĪļÖŹµÄĮæŹĒ£ŗn£ØH2O£©=270 g”Ā18 g•mol-1=15 mol£¬
ŌņŌŚµŚŅ»½×¶ĪŹ§ÖŲ²śĪļÖŠ£ŗn[Al2£ØSO4£©3]£ŗn£ØH2O£©=1 mol£ŗ£Ø18 mol-15 mol£©=1£ŗ3£¬
ŌŚµŚ¶ž½×¶Ī£ŗ
¼ÓČČŗóŹ§Č„µÄĖ®µÄÖŹĮæŹĒ£ŗm£ØH2O£©=666 g”Į£Ø48.65%-40.54%£©=54 g
Ź§Č„½į¾§Ė®µÄĪļÖŹµÄĮæŹĒ£ŗn£ØH2O£©=54 g”Ā18 g•mol-1=3 mol£¬
ŌņŌŚµŚ¶ž½×¶ĪŹ§ÖŲ²śĪļÖŠ½į¾§Ė®ĶźČ«Ź§Č„£¬µĆµ½µÄ¹ĢĢåĪļÖŹŹĒ£ŗAl2£ØSO4£©3£¬ŅŌ“ĖĄ“½ā“š£®
½ā“š ½ā£ŗCu”¢Fe”¢Al”¢AuµÄ»ģŗĻĪļÖŠ¼ÓČėĮņĖįŗĶÅØĻõĖįµÄ»ģŗĻĪļČܽā£¬æÉŅŌµĆµ½ĀĖŅŗ1ĪŖĢśŃĪ”¢ĀĮŃĪ”¢ĶŃĪČÜŅŗ£¬ĀĖŌü1ĪŖAu£¬ĻņĀĖŅŗ1ÖŠ¼ÓČėĖ«ŃõĖ®ŗĶĒāŃõ»ÆÄĘ£¬¼ÓH2O2µÄ×÷ÓĆŹĒ°ŃFe2+Ńõ»ÆĪŖFe3+£¬½įŗĻ³ĮµķµÄpHæÉÖŖ£¬µ÷½ŚpHĪŖ£¼4£¬¹żĀĖ·ÖĄė³öĒāŃõ»ÆĢś£¬ŌŁµ÷½ŚpHĪŖ5×óÓŅ£¬¹żĀĖµĆĀĖŅŗ2ĪŖĮņĖįĶČÜŅŗ£¬ĀĖŌü2ĪŖAl£ØOH£©3£¬¼ÓĮņĖįČܽāŗó½į¾§µĆµ½¾§Ģ壬
£Ø1£©ŌŚ½šŹō»ģŗĻĪļÓėĖįµÄ·“Ó¦ÖŠ£¬ĻõĖį³äµ±Ńõ»Æ¼Į£¬øł¾ŻÉĻĆęµÄ·ÖĪöæÉÖŖĀĖŌü1ĪŖAu£¬¹Ź“š°øĪŖ£ŗAu£»
£Ø2£©²Ł×÷IĪŖÕō·¢”¢ÅØĖõ”¢ĄäČ“½į¾§£¬¹żĀĖ²Ł×÷ĖłÓƵ½µÄ²£Į§ŅĒĘ÷ÓŠÉÕ±”¢²£Į§°ōŗĶĀ©¶·£¬¹Ź“š°øĪŖ£ŗÕō·¢ÅØĖõ£»Ā©¶·£»
£Ø3£©Ąė×Ó²»ČÜÓŚŅŅ“¼£¬ŌņŃ”ÓĆĪŽĖ®ŅŅ“¼µÄŌŅņŹĒ¼õÉŁ¾§ĢåµÄČܽā£¬±ćÓŚøÉŌļ£¬¹Ź“š°øĪŖ£ŗ¼õÉŁ¾§ĢåµÄČܽā£¬±ćÓŚøÉŌļ£»
£Ø4£©µŚ¢Ś²½¼ÓH2O2ŹĒĪŖĮĖ½«Fe2+Ńõ»ÆĪŖFe3+£¬Ąė×Ó·“Ó¦ĪŖ2Fe2++H2O2+2H+ØT2Fe3++2H2O£¬¹Ź“š°øĪŖ£ŗ2Fe2++H2O2+2H+ØT2Fe3++2H2O£»
£Ø5£©ÉčĖłČ”ĮņĖįĀĮ¾§ĢåĪŖ1 mol£¬ŌņĮņĖįĀĮ¾§ĢåµÄÖŹĮæĪŖ666 g£¬
¢ŁŌŚµŚŅ»½×¶Ī£ŗ
¼ÓČČŗóŹ§Č„½į¾§Ė®µÄÖŹĮæŹĒ£ŗm£ØH2O£©=40.54%”Į666 g=270 g£¬
Ź§Č„½į¾§Ė®µÄĪļÖŹµÄĮæŹĒ£ŗn£ØH2O£©=270 g”Ā18 g•mol-1=15 mol£¬
ŌņŌŚµŚŅ»½×¶ĪŹ§ÖŲ²śĪļÖŠ£ŗn[Al2£ØSO4£©3]£ŗn£ØH2O£©=1 mol£ŗ£Ø18 mol-15 mol£©=1£ŗ3£¬
ĖłŅŌ“ĖŹ±µĆµ½µÄ¾§ĢåµÄ»ÆѧŹ½ŹĒ£ŗAl2£ØSO4£©3•3H2O£¬
“š£ŗŹ§ÖŲµŚŅ»½×¶Ī·Ö½ā²śĪļµÄ»ÆѧŹ½ĪŖAl2£ØSO4£©3•3H2O£»
¢ŚŌŚµŚ¶ž½×¶Ī£ŗ
¼ÓČČŗóŹ§Č„µÄĖ®µÄÖŹĮæŹĒ£ŗm£ØH2O£©=666 g”Į£Ø48.65%-40.54%£©=54 g
Ź§Č„½į¾§Ė®µÄĪļÖŹµÄĮæŹĒ£ŗn£ØH2O£©=54 g”Ā18 g•mol-1=3 mol£¬
ŌņŌŚµŚ¶ž½×¶ĪŹ§ÖŲ²śĪļÖŠ½į¾§Ė®ĶźČ«Ź§Č„£¬µĆµ½µÄ¹ĢĢåĪļÖŹŹĒ£ŗAl2£ØSO4£©3£¬
ĖłŅŌµŚ¶ž½×¶Ī·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗAl2£ØSO4£©3•3H2O$\frac{\underline{\;\;”÷\;\;}}{\;}$Al2£ØSO4£©3+3H2O£¬
“š£ŗŹ§ÖŲµŚ¶ž½×¶Ī·“Ó¦µÄ·“Ó¦»Æѧ·½³ĢŹ½ĪŖAl2£ØSO4£©3•3H2O$\frac{\underline{\;\;”÷\;\;}}{\;}$Al2£ØSO4£©3+3H2O£®
µćĘĄ ±¾Ģāæ¼²éĪļÖŹµÄÖʱøŹµŃ飬ĪŖøßĘµæ¼µć£¬°ŃĪÕÖʱøŹµŃéĮ÷³Ģ¼°ĪļÖŹ×é³É”¢ŠŌÖŹ”¢·¢ÉśµÄ·“Ó¦ĪŖ½ā“šµÄ¹Ų¼ü£¬²ąÖŲ·ÖĪöÓėŹµŃ锢¼ĘĖćÄÜĮ¦µÄ漲飬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | ĘĻĢŃĢĒŗĶµ°°×ÖŹµČøß·Ö×Ó»ÆŗĻĪļŹĒČĖĢå±ŲŠčµÄÓŖŃųĪļÖŹ | |
| B£® | ×ŌĄ“Ė®³§ÓĆĆ÷·Æ¾»Ė®£¬Ņ²æÉŅŌÓĆClO2“śĢę | |
| C£® | ¾ÓŹŅÖŠ·ÅÖĆŅ»ÅčŹÆ»ŅĖ®æÉŅŌĪüŹÕCO£¬Ō¤·ĄÖŠ¶¾ | |
| D£® | ÓƶžŃõ»ÆĢ¼Éś²ś¾ŪĢ¼Ėįõ„æÉŅŌ¼õÉŁĢ¼ÅÅ·Å£¬ŅŌ¼õ»ŗĪĀŹŅŠ§Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
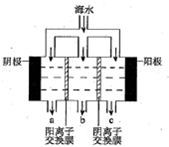 ŗ£Ė®ÖŠÖ÷ŅŖĄė×ÓµÄŗ¬ĮæČēĻĀ£ŗ
ŗ£Ė®ÖŠÖ÷ŅŖĄė×ÓµÄŗ¬ĮæČēĻĀ£ŗ| ³É·Ö | ŗ¬Įæ/£Ømg/L£© | ³É·Ö | ŗ¬Įæ/£Ømg/L£© |
| Cl- | 18980 | Ca2+ | 400 |
| Na+ | 10560 | HCO3- | 142 |
| SO42- | 2560 | Mg2+ | 1272 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
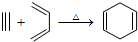 £¬Čē¹ūŅŖŗĻ³É
£¬Čē¹ūŅŖŗĻ³É ĖłÓƵÄŌŹ¼ŌĮĻæÉŅŌŹĒ£Ø””””£©
ĖłÓƵÄŌŹ¼ŌĮĻæÉŅŌŹĒ£Ø””””£©| A£® | 2-¼×»ł-1£¬3-¶”¶žĻ©ŗĶ2-¶”Č² | B£® | 1£¬3-Īģ¶žĻ©ŗĶ2-¶”Č² | ||
| C£® | 2£¬3-¶ž¼×»ł-1£¬3-Īģ¶žĻ©ŗĶŅŅČ² | D£® | 2£¬3-¶ž¼×»ł-1£¬3-¶”¶žĻ©ŗĶ1-¶”Č² |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
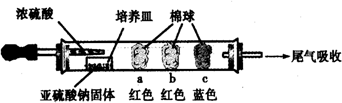
| ĆŽĒņ | ĆŽĒņÉĻµĪ¼ÓµÄŹŌ¼Į | ŹµŃéĻÖĻó | ½āŹĶŗĶ½įĀŪ |
| a | Ę·ŗģŹŌŅŗ | ĆŽĒņ±ä°×£¬Ī¢ČČŗóÓÖ»Öø“ŗģÉ« | SO2¾ßÓŠĘư׊Ō£¬ĒŅĪŖŌŻŹ±ŠŌĘÆ°× |
| b | ŗ¬·ÓĢŖµÄNaOHČÜŅŗ | ĆŽĒņ±äĪŖ°×É« | Ąė×Ó·½³ĢŹ½£ŗ2OH”„+SO2 =SO32”„+H2O»ņOH”„+SO2=HSO3”„ |
| c | ŗ¬µķ·ŪµÄµāĖ® | ĆŽĒņ±äĪŖ°×É« | øĆĘųĢå¾ßÓŠ»¹ŌŠŌ £ØŃ”Ģī”°Ńõ»ÆŠŌ”±»ņ”°»¹ŌŠŌ”±£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com