
 53������ϵ�д�
53������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ��ˮ��ѧ����12�½��Բ��Ի�ѧ�Ծ����������� ���ͣ������
���Ļ������ж�������+6��Cr��ǿ�����ԣ��䶾����+3��Cr���Ե�100������ˣ�����Ժ����ķ�ˮ���д�����Ŀǰ�о��Ͳ��õĴ���������Ҫ�У�
����һ����ԭ����
���������Խ�������FeSO4��NaHSO3�Ƚ�+6��Cr��ԭ��+3��Cr�������������£�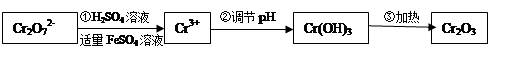
��ش��������⣺
��1���������̢ٷ�����Ӧ�����ӷ���ʽ�� ��
��2�����ڢ� ʹFeSO4�ʵ������������������ɲ������д��ԡ���������������壨Fe3O4��FeO��Fe2O3���ĸ�������� ��y
��y O��(ע��X�����Ƿ���)�����Ϊ��������ƿ���������Ŀ���� ��ʹ�������������ϼ��ķ����� �����账����1 mol Cr2O72���ķ�ˮ������Ҫ����10mol FeSO4��7H2O�������
O��(ע��X�����Ƿ���)�����Ϊ��������ƿ���������Ŀ���� ��ʹ�������������ϼ��ķ����� �����账����1 mol Cr2O72���ķ�ˮ������Ҫ����10mol FeSO4��7H2O������� ��y
��y O����ѧʽ�� ��
O����ѧʽ�� ��
�����о����֣�����������ԭ���������Գ�ȥCr6+�����ܳ�ȥ��ˮ�е�����Mn2+�����о���м������pHֵ�Է�ˮ�и�����ȥ���ʵ�Ӱ�죬
��3��ȡ100mL��ˮ��250 mL����ƿ�У�����pHֵ���涨ֵ���ֱ���벻ͬ���ķ���м���õ���м�����Ը�����ȥ���ʵ�Ӱ������ͼ1��ʾ������pHһ��ʱ����ˮ����м����Ϊ ʱ�̡���ȥ�������
��4��ȡ100mL��ˮ��250 mL����ƿ�У�����涨�������ۣ����ɲ�ͬ��pHֵ���õ�pHֵ�Ը�����ȥ���ʵ�Ӱ������ͼ2��ʾ��������м����һ��ʱ����ˮpH= ʱ�̡���ȥ�������

����������ⷨ������+6��Cr�ķ�ˮ��������ڣ������������������������Ȼ��ƽ��е�⣺���������ɵ�Fe2+��Cr2O72-������Ӧ�����ɵ�Fe3+��Cr3ʮ����������OHһ�������Fe��OH��3��Cr��OH��3������ȥ��
��5��д����������Ӧ�ĵ缫����ʽ ��
��6�������Ϸ�����1��104 L������+6�ۣ�78 mg / L�ķ�ˮ�����ʱ�����������ĵ���������Ϊ________kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ����12�½��Բ��Ի�ѧ�Ծ��������棩 ���ͣ������
���Ļ������ж�������+6��Cr��ǿ�����ԣ��䶾����+3��Cr���Ե�100������ˣ�����Ժ����ķ�ˮ���д�����Ŀǰ�о��Ͳ��õĴ���������Ҫ�У�
����һ����ԭ����
���������Խ�������FeSO4��NaHSO3�Ƚ�+6��Cr��ԭ��+3��Cr�������������£�
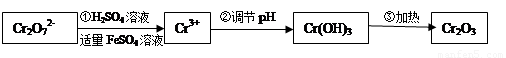
��ش��������⣺
��1���������̢ٷ�����Ӧ�����ӷ���ʽ�� ��
��2�����ڢ� ʹFeSO4�ʵ������������������ɲ������д��ԡ���������������壨Fe3O4��FeO��Fe2O3���ĸ�������� ��y
��y O��(ע��X�����Ƿ���)�����Ϊ��������ƿ���������Ŀ���� ��ʹ�������������ϼ��ķ����� �����账����1 mol Cr2O72���ķ�ˮ������Ҫ����10mol
FeSO4��7H2O�������
O��(ע��X�����Ƿ���)�����Ϊ��������ƿ���������Ŀ���� ��ʹ�������������ϼ��ķ����� �����账����1 mol Cr2O72���ķ�ˮ������Ҫ����10mol
FeSO4��7H2O������� ��y
��y O����ѧʽ�� ��
O����ѧʽ�� ��
�����о����֣�����������ԭ���������Գ�ȥCr6+�����ܳ�ȥ��ˮ�е�����Mn2+�����о���м������pHֵ�Է�ˮ�и�����ȥ���ʵ�Ӱ�죬
��3��ȡ100mL��ˮ��250 mL����ƿ�У�����pHֵ���涨ֵ���ֱ���벻ͬ���ķ���м���õ���м�����Ը�����ȥ���ʵ�Ӱ������ͼ1��ʾ������pHһ��ʱ����ˮ����м����Ϊ ʱ�̡���ȥ�������
��4��ȡ100mL��ˮ��250 mL����ƿ�У�����涨�������ۣ����ɲ�ͬ��pHֵ���õ�pHֵ�Ը�����ȥ���ʵ�Ӱ������ͼ2��ʾ��������м����һ��ʱ����ˮpH= ʱ�̡���ȥ�������


����������ⷨ������+6��Cr�ķ�ˮ��������ڣ������������������������Ȼ��ƽ��е�⣺���������ɵ�Fe2+��Cr2O72-������Ӧ�����ɵ�Fe3+��Cr3ʮ����������OHһ�������Fe��OH��3��Cr��OH��3������ȥ��
��5��д����������Ӧ�ĵ缫����ʽ ��
��6�������Ϸ�����1��104 L������+6�ۣ�78 mg / L�ķ�ˮ�����ʱ�����������ĵ���������Ϊ________kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����϶���������������������ʸߣ�95%���ϣ�����ʡ���͡������������ͺͼ���û��β����Ⱦ���������ܣ��ܵ�Խ��Խ��Ĺ�ע��ij�ֻ�϶��������Ķ���ϵͳ�ɡ�1.3L���ͻ�+5���ֶ�������+10kW���+144V�����ء���ɡ�
�����ͻ���ȼ��֮һ���Ҵ���1g�Ҵ���ȫȼ�շų�29.7kJ������д���Ҵ�ȼ�յ��Ȼ�ѧ����ʽ___________________________________��
�������й��ʹ�ÿɼ����ؽ���������Ⱦ�������ô��������Ϊ��������Һ����Ҫ��KOH����Ϊ���Һ�������س��ʱ������Ӧ��Ni(OH)2=== Ni(OH)+1/2H2����ŵ�ʱ�����缫��ӦʽΪ__________________________________��
�۳����£�Ũ��ͬΪ0.1mol��L-l��NaHCO3��Һ��Na2CO3 ��ҺpH ֵ������7 ��������_______ pH������ԭ���ǣ�___________________________________��
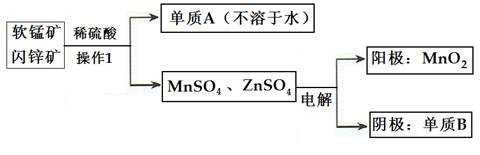
(2)�������̡�п������ɵ�ص���Ҫԭ�ϣ���ҵ�������̿�(��MnO2)����п��(��ZnS)���������������̡�п�Ĺ������£�
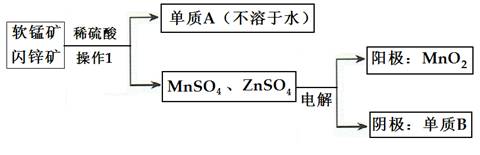
�ٲ���1������Ϊ___________������AΪ____________������BΪ______________��
�������������У�����������6.5gBʱ��������MnO2����Ϊ_________________��
���������ȷ�Ӧԭ�����ɴ����̿�����ȡ�����̣�д���仯ѧ��Ӧʽ��__________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����϶���������������������ʸߣ�95%���ϣ�����ʡ���͡������������ͺͼ���û��β����Ⱦ���������ܣ��ܵ�Խ��Խ��Ĺ�ע��ij�ֻ�϶��������Ķ���ϵͳ�ɡ�1.3L���ͻ�+5���ֶ�������+10kW���+144V�����ء���ɡ�
�����ͻ���ȼ��֮һ���Ҵ���1g�Ҵ���ȫȼ�շų�29.7kJ������д���Ҵ�ȼ�յ��Ȼ�ѧ����ʽ___________________________________��
�������й��ʹ�ÿɼ����ؽ���������Ⱦ�������ô��������Ϊ��������Һ����Ҫ��KOH����Ϊ���Һ�������س��ʱ������Ӧ��Ni(OH)2=== Ni(OH)+1/2H2����ŵ�ʱ�����缫��ӦʽΪ__________________________________��
�۳����£�Ũ��ͬΪ0.1mol��L-l��NaHCO3��Һ��Na2CO3 ��ҺpH ֵ������7 ��������_______ pH������ԭ���ǣ�___________________________________��
(2)�������̡�п������ɵ�ص���Ҫԭ�ϣ���ҵ�������̿�(��MnO2)����п��(��ZnS)���������������̡�п�Ĺ������£�
�ٲ���1������Ϊ___________������AΪ____________������BΪ______________��
�������������У�����������6.5gBʱ��������MnO2����Ϊ_________________��
���������ȷ�Ӧԭ�����ɴ����̿�����ȡ�����̣�д���仯ѧ��Ӧʽ��__________________________________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com