
���� ����ʵ��ԭ����֪����NH4HCO3��NaHSO3�Ļ�����м������������̼�Ͷ����������壬�ø��������Һ��ȥ��������Ȼ��ͨ��Ʒ����Һ������������Ƿ���������Ž�����ͨ��Ũ����������ü�ʯ�����ն�����̼���壬��ʯ�����ӵ�������Ϊ������̼��������������ݶ�����̼�������ɼ����������NH4HCO3������ȷ��NH4HCO3�ĺ�����
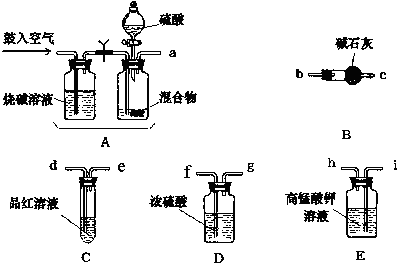
��1������װ��ͼ��֪�������ƣ�
��2��ʵ�鿪ʼ����������ǰ��Aװ����Ҫ�������Ŀ�����ų�ϵͳ�еĶ�����̼���壬������ٴι������������
��ʹ���ɵ�����ȫ���������װ�ã�
��3�����������Һ�����������ն����������壮
��4����Ʒ����Һ��ɫ��˵��������������û�г�����������ʯ�����գ����Լ�ʯ��������ƫ���ٸ���ʵ��ԭ��������
��5����ʯ������4.4g��������̼���ʵ���Ϊ0.1mol����̼Ԫ���غ㣬̼��������ʵ���ҲΪ0.1mol��̼���������Ϊ7.9g��������NH4HCO3������������
��6����ʯ�������տ����еĶ�����̼��ˮ������
��� �⣺��1������װ��ͼ��֪A�м���������Һ�����������Ƿ�Һ©�����ʴ�Ϊ����Һ©����
��2��ʵ�鿪ʼ����������ǰ��Aװ����Ҫ�������Ŀ�����ų�ϵͳ�еĶ�����̼���壬������ٴι��������������ʹ���ɵ�����ȫ���������װ�ã��ʴ�Ϊ�������ɵ�����ȫ���������װ�ã�
��3�����������Һ�����������ն����������壬�ʴ�Ϊ�����ն����������壻
��4����Ʒ����Һ��ɫ��˵��������������û�г�����������ʯ�����գ����Լ�ʯ��������ƫ����ʵ��ԭ��������֪������̼���������ƫ����������NH4HCO3ƫ�ⶨ�����ƫ�ߣ��ʴ�Ϊ���ߣ�
��5����ʯ������4.4g����������̼���ʵ���Ϊ0.1mol����̼Ԫ���غ㣬̼��������ʵ���ҲΪ0.1mol��̼���������Ϊ7.9g��NH4HCO3����������Ϊ$\frac{7.9g}{13.1g}$��100%=60.3%���ʴ�Ϊ��60.3%��
��6����ʯ���������ͨ����ʯ�������տ����еĶ�����̼��ˮ����������Ӧ�ڼ�ʯ�Һ���������һ��װ�м�ʯ�ҵ�װ�ã�
�ʴ�Ϊ����B֮���ٽ�һ����ֹ������ˮ�Ͷ�����̼����B��װ�ã�
���� ������ҪĿ���Dzⶨ������̼���������������������Ҳ�ܱ���ʯ�����գ����������ն�����̼����ǰӦ���������������������ȥ������������һ�������Ը��������Һ����ˮ������Ʒ����Һ����������������Ƿ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ͬ��Ԫ�أ����ź�����Ӳ��������ӣ�I1������ | |
| B�� | ͨ������£�����ͬһ��Ԫ�ص�ԭ�ӣ��������I1��I2��I3 | |
| C�� | ͬ����Ԫ�أ�����仯���������ź˵���������ӣ�I1���� | |
| D�� | ͨ������£�������ԽС��Ԫ�صĽ�����Խǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | ���� | C�� | ��С | D�� | ���ж� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����¶ȣ�����Ӧ���ʼ�С������Ӧ�������� | |
| B�� | �����¶������������淴Ӧ�������Ӷ����̴ﵽƽ���ʱ�� | |
| C�� | �ﵽƽ��������¶Ȼ�����ѹǿ�������ڸ÷�Ӧƽ�������ƶ� | |
| D�� | �ﵽƽ������¶Ȼ��Сѹǿ�������ڸ÷�Ӧƽ�������ƶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 ij��ѧ�С�����ʵ�飨װ����ͼ����֤�ȡ��塢�⼰�仯������й����ʣ���Ӳ�ʲ������е�A��B��C�������η���ʪ�����ɫʯ����ֽ������NaBr��Һ�������е���-KI��Һ��������ͼ��ʾ���������ͨ�������������ش��������⣺
ij��ѧ�С�����ʵ�飨װ����ͼ����֤�ȡ��塢�⼰�仯������й����ʣ���Ӳ�ʲ������е�A��B��C�������η���ʪ�����ɫʯ����ֽ������NaBr��Һ�������е���-KI��Һ��������ͼ��ʾ���������ͨ�������������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2H2O$\frac{\underline{\;ͨ��\;}}{\;}$ 2H2��+O2 | B�� | 2Na+2H2O�T2NaOH+H2�� | ||
| C�� | Cl2+H2O�THC1+HC1O | D�� | 2F2+2H2O�T4HF+O2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com