
����Ŀ��N��Fe��������Ҫ��Ԫ�أ��䵥�ʼ�����������������ж��й㷺��Ӧ�á�
(1)��̬Nԭ������ܼ��ĵ���������ͼ��״��__________���������______�ֲ�ͬ�˶�״̬�ĵ��ӡ�
(2)��һ������N_____O�����������������������ԭ����_______________________��
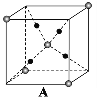
(3)�ڸ�ѹ�µ����ᷢ���ۺϵõ��߾۵���������ÿ����ԭ��������������ԭ�ӽ���γɿռ���״�ṹ���߾۵��ľ���������__________����ԭ�ӵ��ӻ��������Ϊ__________��
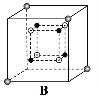
(4)����������BN����ʯī����ṹ���ƣ���ԭ�Ӻ͵�ԭ�ӽ�����������ʯī���Ե��������BNȴ���ܵ��磬��ԭ����_____________________________��
(5)�����ᣨHN3��������������������ҪӦ�á������ᣨHN3������HNO2�����£�N2H4���Ƶã���ѧ����ʽ��N2H4 + HNO2��HN3 + 2H2O�����������������_________��
A��HN3��N2H4�����ɼ��Լ��ͷǼ��Լ����ɵķǼ��Է���
B��NaN3�ľ����ܴ���KN3�ľ�����
C��HN3�������ĸ�ԭ�ӿ�����һ��ֱ����
D�������ᣨHN3����ˮ���γɷ��Ӽ����
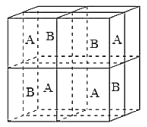
(6)ij�����������������ᄃ����ͼ��ʾ������A��B������ɡ���û�������Fe2����Fe3����O2���ĸ�������__________������������ȣ�����֪�þ���ľ�������Ϊa nm�������ӵ�������ֵΪNA����þ�����ܶ���_______ g![]() cm��3���ú�a��NA�Ĵ���ʽ��ʾ����
cm��3���ú�a��NA�Ĵ���ʽ��ʾ����
![]()
���𰸡��Ĵ��Σ��������Σ� 7 �� Nԭ�ӵ�2p���Ϊ���ȶ��İ�����ṹ����Oԭ��ʧȥһ�����Ӻ�2p�����Ϊ���ȶ��İ�����ṹ������Nԭ�ӵĵ�һ�����ܴ���Oԭ�� ԭ�Ӿ��� sp3 BN��Nԭ�ӵ縺�Դ�ʹNԭ��2p����ϵĵ��ӶԱ�������Nԭ���ϣ����������ƶ�����˲����� AC 1��2��4 ![]()
��������
��1��Nԭ�Ӻ˵����Ϊ7����̬N ԭ������ܼ�Ϊ2p�ܼ���
��2��Nԭ�ӵ�p���Ϊ������Ƚ��ȶ�����ԭ�ӱ���ԭ������ʧȥ���ӣ�
��3��������ÿ����ԭ��������������ԭ�ӽ���γɿռ���״�ṹ���ɴ��жϾ������ͣ�ÿ��Nԭ�Ӻ���һ���µ��Ӷԣ����ݼ۲���ӶԻ��������жϵ�ԭ�ӵ��ӻ�������ͣ�
��4����Ϊ���ĵ縺�Խϴ��ƽ�����ص�p���������������ϵĵ����ںܴ�̶��ϱ������ڵ�����Χ�����������ƶ���
��5��A��HN3��N2H4�м��м��Թ��ۼ���Ҳ�зǼ��Թ��ۼ���B��NaN3��KN3Ϊ�ṹ���Ƶ����Ӿ��壬Na+��K+�����ͬ��Na+�뾶С��K+���뾶ԽС��������Խ��C��HN3��N3-Ϊֱ�߽ṹ����H������NΪsp2�ӻ���D��HN3��Ҳ���ڵ縺�Խϴ��N��
��6��Fe2+���Ӵ��ھ����Ķ��㡢�����Լ�Aλ������������ġ�O2-λ��A��BС��������ڲ���ÿ��С�������ڲ�����4����Fe3+���Ӵ��ھ���Bλ��С�������ڲ�����̯�����㾧����Fe2+��Fe3+��O2-�ĸ��������㾧����������Ͼ�������=�����ܶ�������������㾧���ܶȡ�
��1������7��Ԫ�أ����ڵڶ�����VA�壬��������Ų�ʽΪ1s22s22p3������ܼ�Ϊ2p����������״Ϊ�����Σ�����7�������˶�״̬����ͬ������7�ֲ�ͬ�˶�״̬�ĵ��ӡ�
��2��Oԭ�ӵļ۵����Ų�Ϊ2s22p4��Nԭ�ӵļ۵����Ų�Ϊ2s22p3��p���Ϊ������Ƚ��ȶ�����ԭ�ӱ���ԭ������ʧȥ���ӣ��ʵ�Ԫ�صĵ�һ�����ܴ�����Ԫ�صģ�
��3��������ÿ����ԭ��������������ԭ�ӽ���γɿռ���״�ṹ����˾���Ϊԭ�Ӿ��壻ÿ��N�γ�3��N-N����������1�Թµ��Ӷԣ��ӻ������ĿΪ4��Nԭ���ӻ���ʽΪsp3��
��4����Ϊ���ĵ縺�Խϴ��ƽ�����ص�p���������������ϵĵ����ںܴ�̶��ϱ������ڵ�����Χ�����������ƶ�����������BN�����磻
��5��A��HN3��N2H4�м��м��Թ��ۼ���Ҳ�зǼ��Թ��ۼ�������ǰ���Ǽ��Է��ӣ������ǷǼ��Է��ӣ���A����B��NaN3��KN3Ϊ�ṹ���Ƶ����Ӿ��壬Na+��K+�����ͬ��Na+�뾶С��K+���뾶ԽС��������Խ����NaN3�ľ����ܴ���KN3�ľ����ܣ���B��ȷ��C��HN3��N3-Ϊֱ�߽ṹ����H������NΪsp2�ӻ��������ĸ�ԭ�Ӳ�������ͬһֱ���ϣ���C����D��HN3��Ҳ���ڵ縺�Խϴ��N����ˮ���γɷ��Ӽ��������D��ȷ���ʴ�ΪAC��
��6��Fe2+���Ӵ��ھ����Ķ��㡢�����Լ�Aλ������������ġ�O2-λ��A��BС��������ڲ���ÿ��С�������ڲ�����4����Fe3+���Ӵ��ھ���Bλ��С�������ڲ���������Fe2+������Ŀ=4+8��![]() +6��
+6��![]() =8��Fe3+������Ŀ=4��4=16��O2-������Ŀ=4��8=32����Fe2+��Fe3+��O2-�ĸ�����Ϊ8:16:32=1:2:4��Fe��Oԭ����Ŀ֮��=24:32=3:4���������ﻯѧʽΪFe3O4�������൱����8����Fe3O4������������=8��
=8��Fe3+������Ŀ=4��4=16��O2-������Ŀ=4��8=32����Fe2+��Fe3+��O2-�ĸ�����Ϊ8:16:32=1:2:4��Fe��Oԭ����Ŀ֮��=24:32=3:4���������ﻯѧʽΪFe3O4�������൱����8����Fe3O4������������=8��![]() g����������Ϊa nm����8��
g����������Ϊa nm����8��![]() g=��g
g=��g![]() cm��3����a��10-7 cm��3�������=
cm��3����a��10-7 cm��3�������=![]() g
g![]() cm��3��
cm��3��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ����0.1mol/LNaA��Һ�еεμ����ᣬ���û����Һ��pH��![]() �ı仯��ϵ����ͼ��ʾ��
�ı仯��ϵ����ͼ��ʾ��![]() ����������ȷ����
����������ȷ����
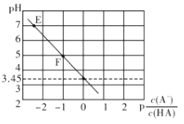
A. E����Һ��c(Na+)��c(A��)
B. Ka(HA)��������Ϊ10��3
C. �μӹ�����![]() ���ֲ���
���ֲ���
D. F����Һ��c(Na+)��c(HA)��c(A��)��c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��CΪԭ�Ϻϳ����ƶ��Ӳ��֢ҩ��H��·�����£�
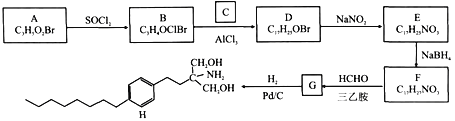
��֪��
��A����NaHCO3��Һ��Ӧ�ų�CO2����˴Ź���������ʾ������壬�������Ϊ2:2:1��
��NaBH4��ѡ���Ի�ԭȩ��ͪ��������ԭ��NO2��

�ش��������⣺
(1)A�Ļ�ѧ����Ϊ________��D�Ľṹ��ʽΪ_______��
(2)H�ķ���ʽΪ_______��E�й����ŵ�����Ϊ_______��
(3)B��D��E��F�ķ�Ӧ���ͷֱ�Ϊ_______��
(4)F��G�Ļ�ѧ����ʽΪ________��
(5)��C��Ϊͬ���칹����л���Ľṹ��ʽΪ_______(�˴Ź�������Ϊ����壬�������Ϊ6:3:1:1)��
(6)�����B��![]() Ϊԭ���Ʊ����п���������ҩ��
Ϊԭ���Ʊ����п���������ҩ�� �ĺϳ�·��__________��
�ĺϳ�·��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���۲�����ʵ��װ��ͼ����Ҫ������

��1��д��ͼ������ʵ����������ƣ�___________________________________________________��
��2��װ�â������в������������ƣ�____________��____________��
��3������ʵ����Ҫ������װ���н��У�������ţ�ÿ��װ�ý�ʹ��һ�Σ�
A�Ӻ�ˮ�л�ȡ����ˮ____________��B��KCl��Һ�л�ȡKCl����____________��
C����CaCO3��ˮ____________��D����ֲ���ͺ�ˮ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������һ�仰������������ֵ��ǰ��Ϊ![]() ������Ϊ
������Ϊ![]() ��
��![]() ��
��![]() �Ĺ�ϵ��A��B��C��D��ѡ����ա�
�Ĺ�ϵ��A��B��C��D��ѡ����ա�
A.![]() B.
B.![]() C.
C.![]() D.���Ƚ�
D.���Ƚ�
��1��������![]() ��
��![]() ��
��![]()
![]() �������Ϻ���Һ��
�������Ϻ���Һ��![]() ��
��![]() ��______��
��______��
��2��ͬ�¶��£�![]()
![]() ��Һ��
��Һ��![]() ˮ��ٷ�����
ˮ��ٷ�����![]() ��Һ��
��Һ��![]() ��ˮ��ٷ��ʣ�______��
��ˮ��ٷ��ʣ�______��
��3��pHֵ��ͬ�Ĵ�������ᣬ�ֱ�������ˮϡ����ԭ����![]() ����
����![]() ����ϡ�ͺ�����Һ��
����ϡ�ͺ�����Һ��![]() ֵ��Ȼ��ͬ����
ֵ��Ȼ��ͬ����![]() ��
��![]() �Ĺ�ϵ�ǣ�______��
�Ĺ�ϵ�ǣ�______��
��4�����������ݵ�Ũ�ȵĴ�����Һ�����ڶ��������¶ȣ�����Һ��![]() ��______��
��______��
��5����ͬ�¶��£�![]() ֵΪ12���ռ���Һ��ˮ�ĵ���Ⱥ�
ֵΪ12���ռ���Һ��ˮ�ĵ���Ⱥ�![]() ֵΪ12��
ֵΪ12��![]() ��Һ��ˮ�ĵ���ȣ�______��
��Һ��ˮ�ĵ���ȣ�______��
��6����![]() ֵΪ2������ʹ��ᶼϡ����ͬ��������ϡ��Һ��
ֵΪ2������ʹ��ᶼϡ����ͬ��������ϡ��Һ��![]() ֵ��______��
ֵ��______��
��7��������ijǿ���ijǿ����Һ�������Ϻ���Һ��![]() ֵΪ7��ԭ����Һ��ԭ����Һ�����ʵ���Ũ�ȣ�______��
ֵΪ7��ԭ����Һ��ԭ����Һ�����ʵ���Ũ�ȣ�______��
��8����ͬ�¶��£�![]() ��
��![]() ��Һ�е�
��Һ�е�![]() ������
������![]()
![]() ��Һ��
��Һ��![]() �ĸ�����______��
�ĸ�����______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��C(s)��O2(g)��CO2(g) ��H1
CO2(g)��C(s)��2CO(g) ��H2
2CO(g)��O2(g)��2CO2(g) ��H3
4Fe(s)��3O3(g)��2Fe2O3(s) ��H4
3 CO(g)��Fe2O3(s)��3CO2(g)��2Fe(s) ��H5
���й���������Ӧ�ʱ���ж���ȷ����
A. ��H1��0����H3��0
B. ��H2��0����H4��0
C. ��H1����H2����H3
D. ��H3����H4����H5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����չ��̼һ��ѧ�������������ҹ��ḻ��ú̿��Դ������Ҫ��ս������;��ü�ֵ����ش��������⣺
(1)��֪��������C(s)��ȼ���ȡ�H=-393.5 kJ��mol-1�� S(s)��ȼ��ȡ�H=-296.0 kJ��mol-l ��CO2(g)+C(S)=2CO(g) ��H=+172.5 kJ��mol-1��д��һ����̼����������ԭΪ��������Ȼ�ѧ����ʽ��______
(2)��763 K��3.04��104 kPaʱ����CO��H2��ԭ�Ϻϳɼ״�(CH3OH)����������ƽ�⣺CO(g)+2H2(g) ![]() CH3OH(g)����ԭ����CO��H2�ı�����ͬʱ����CO��ת���ʼ�ƽ�������м״��������������Ӱ�졣
CH3OH(g)����ԭ����CO��H2�ı�����ͬʱ����CO��ת���ʼ�ƽ�������м״��������������Ӱ�졣
����H2��CO��ʼ���ʵ���֮��Ϊm��ƽ��ʱCO��ת����Ϊ����ƽ�������м״����������Ϊy����m������y���ߵĹ�ϵʽΪy=___��
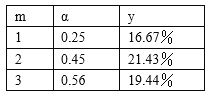
�ڸ��ݱ����ṩ�����ݣ��ɵó���Ӧ��ı�����CO��ƽ��ת�����Լ�ƽ�������м״����������Ӱ��Ľ��ۣ�ѡ����ѷ�Ӧ�����m=_______������l������2������3������������_________��
(3)��ͼ�����ֽ��������ﱻһ����̼��ԭ����Ӧ�ﵽƽ��ʱlgc(CO)/c(CO2)���¶�(T)�Ĺ�ϵ����ͼ��

��8000Cʱ���������ױ���ԭ�Ľ�����������______���ѧʽ�����÷�Ӧ��ƽ�ⳣ��K=_______��
��CO2��ԭPbO2�ķ�Ӧ��H ___0������>������<��)���ж�������_________��
(4)��ѧ�������о��ù�̬������Ϊ����ƽ����������ƽ���������غ����ǵĻ�����ȼ����ȼ������ȼ�գ�������ӦKNO3+C12H22O11��CO2��+N2��+H2O+K2CO3����δ��ƽ������÷�Ӧ���������뻹ԭ�������ʵ���֮����_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ����Ũ�ȶ�Ϊ1 mol��L-1�ģ�NH4��2SO4��NH4H CO3��NH4HSO4��NH4Cl��������Һ�У����c��NH4+���ֱ�Ϊa��b��c��d����λΪmol��L-1���������ж���ȷ����
A. a=2b=2c=2d B. a>b>c> d C. a>c>d>b D. a>c>b>d
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijԪ�ص�һ��ͬλ��X��ԭ��������ΪA����N�����ӣ�����![]() ԭ�����HmX���ӣ���a��HmX���������ӵ����ʵ����ǣ� ��
ԭ�����HmX���ӣ���a��HmX���������ӵ����ʵ����ǣ� ��
A. ![]() (A-N+m)molB.
(A-N+m)molB. ![]() (A-N)molC.
(A-N)molC. ![]() (A-N)molD.
(A-N)molD. ![]() (A-N+m)mol
(A-N+m)mol
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com