

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ƾ���ˮ | B��������Ȼ�̼ | C��ˮ�����Ȼ�̼ | D��ʳ��ˮ����ˮ |
�鿴�𰸺ͽ���>>
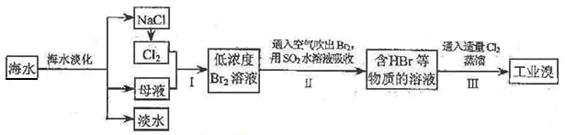
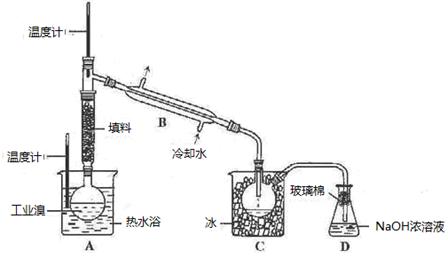
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
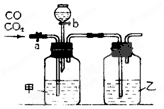
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �������£�
�������£� �ϸ�ƿ��ʢ�ŵ��Լ�Ϊ���û�ѧʽ�ش𣩣���ÿ��1�֣�
�ϸ�ƿ��ʢ�ŵ��Լ�Ϊ���û�ѧʽ�ش𣩣���ÿ��1�֣� ��D ��
��D ��
 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 B����
B�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����Լ���SO����BaCl2��Һ��HNO3�ữ |
B��Ϊ��߸������ ��Һ�����������������Ὣ���������Һ�ữ ��Һ�����������������Ὣ���������Һ�ữ |
| C��������Һ���Ƿ���Fe2��ʱ���������ữ |
| D��������Һ���Ƿ���SOʱ���������������Ӹ��ŵ������£����������ữ��������Һ�ټ�BaCl2��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��OH?����ɫʯ����ֽ�� | B��Cl?�������ữ��AgNO3�� |
| C��I2�����ۣ� | D��H+ ����̪�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Cu��Fe��----��ϡ���ᣬ���� |
| B��CO2��SO2��----����������Һ��ϴ���� |
| C��NaCl��Һ���⣩----�ƾ�����ȡ����Һ�� |
| D��KNO3��Һ��KCl��----���½ᾧ�����ˣ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com