
| A��H2C2O4��2H2O | B��FeSO4 | C��Ũ���� | D��Na2SO3 |
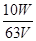 ��Һ����ɫ��dz��ɫ���Ұ�����ڲ���ɫ��(4)ƫ�� �����л�ԭ�����ʵ����ã�ʹKMnO4��Һ��Ũ�ȱ�С�����¼��������c(Fe2��)������ 5Fe2����MnO4-��8H��=5Fe3����Mn2����4H2O
��Һ����ɫ��dz��ɫ���Ұ�����ڲ���ɫ��(4)ƫ�� �����л�ԭ�����ʵ����ã�ʹKMnO4��Һ��Ũ�ȱ�С�����¼��������c(Fe2��)������ 5Fe2����MnO4-��8H��=5Fe3����Mn2����4H2O 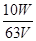 mol��L��1���ζ��յ���ɫ�仯Ϊ��Һ����ɫ��dz��ɫ���Ұ�����ڲ���ɫ����4��KMnO4����Һ���ã������ױ������л�ˮ�е�ijЩ������ԭ�����ʻ�ԭ����ʹŨ�Ƚ��ͣ��ⶨˮ����Fe2���ĺ�������õ�Ũ��ֵ��ƫ�ߣ����������������Һ�о���ǿ��������������������Ϊ�����ӣ���������ԭΪ�����ӣ����������ӷ�ӦΪ��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O
mol��L��1���ζ��յ���ɫ�仯Ϊ��Һ����ɫ��dz��ɫ���Ұ�����ڲ���ɫ����4��KMnO4����Һ���ã������ױ������л�ˮ�е�ijЩ������ԭ�����ʻ�ԭ����ʹŨ�Ƚ��ͣ��ⶨˮ����Fe2���ĺ�������õ�Ũ��ֵ��ƫ�ߣ����������������Һ�о���ǿ��������������������Ϊ�����ӣ���������ԭΪ�����ӣ����������ӷ�ӦΪ��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Һʱ����Һ©���²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� |
| B������ʱ��Ӧʹ�¶ȼ�ˮ����������ƿ֧�ܿ� |
| C�������ᾧʱ��������ʣ������Һ��ʱ��ֹͣ���ȣ��������Ƚ�Һ������ |
| D��������ƿ����һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���ܽ�����ҺҪ����ת��������ƿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
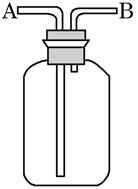
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
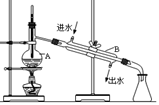
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ʵ�����ģ�ʡ�Լг�װ�ã� | ��Ӧʵ�� |
| A | �Թܡ���ͷ�ι� | ��ϡ���ᡢNa2CO3��Һ�Ƚ�������̼�������ǿ�� |
| B | �ձ�������������ͷ�ιܡ���ֽ | �������ȥ���ᱵ�е�����̼�ᱵ |
| C | �ձ�������������ͷ�ιܡ�����ƿ | �ù����Ȼ�������0.5mol/L����Һ |
| D | �ձ����������������� | ����ͭ��Һ��Ũ���ᾧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
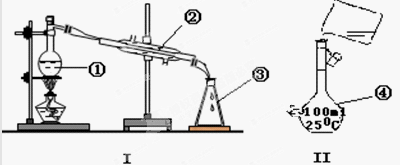
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ�����ģ�ʡ�Լг�װ�ã� | ��Ӧʵ�� |
| A | �ձ����������������� | 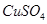 ��Һ��Ũ���ᾧ ��Һ��Ũ���ᾧ |
| B | �ձ�������������ͷ�ιܡ���ֽ | �������ȥ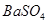 �� ��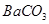 |
| C | �ձ�������������ͷ�ιܡ�����ƿ | �ù���NaCl����0.5mol/L����Һ |
| D | �ձ�������������ͷ�ιܡ���Һ©�� | ����ˮ��CCl4,��ȥ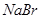 ��Һ������ ��Һ������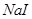 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com