

| ||
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ò�˿պȡij��Һ���ھƾ��ƻ��������գ�ֱ�ӹ۲������ɫ������K+�Ĵ��� |
| B���ò�����պȡNa2CO3��Һ�����ڸ����pH��ֽ�ϣ��ⶨ����Һ��pH |
| C�����ȵļ���Һϴ��𤸽���Թܱ��ϵ���֬ |
| D������һ�����ʵ���Ũ�ȵ���Һ��������ƿ��ˮ��Һ����̶���1��2cmʱ�����ý�ͷ�ιܶ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ʾ��װ����ʵ��������;�ȽϹ㷺��
��ͼ��ʾ��װ����ʵ��������;�ȽϹ㷺���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
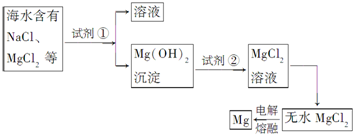
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ʳ�ﴢ���ڱ����� |
| B����H2O2��Һ�м��뼸��FeCl3��Һ |
| C���ÿ�״̼��ƴ����ĩ״̼�����ϡ���ᷴӦ |
| D����0.1 mol/L H2SO4��Һ����1 mol/L H2SO4��Һ��п����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��4g | B��4.5g |
| C��5g | D��5.5g |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com