
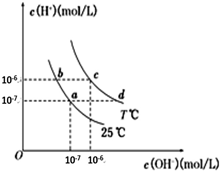
| A��a���Ӧ����Һ��c���Ӧ����ҺpHֵ��С��pH��c����pH��a�� | B��d���Ӧ����Һ�д������ڣ�K+��Ba2+��NO3-��I- | C��25��ʱ��Ka��HF��=3.6��10-4��Ka��CH3COOH��=1.75��10-5��0.1mol/L��NaF��Һ��0.1mol/L ��CH3COOK��Һ��ȣ�c��Na+��-c��F-����c��K+��-c��CH3COO-�� | D����b���Ӧ����Һ��ֻ��NaHA������Һ������Ũ�ȴ�С��c��HA-����c��H2A����c��H+����c��A2-�� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ڲ�ͬ�¶��µ�ˮ��Һ��c��H+��=10x mol/L��c��OH-��=10y mol/L��x��y�Ĺ�ϵ��ͼ��ʾ����ش��������⣺
�ڲ�ͬ�¶��µ�ˮ��Һ��c��H+��=10x mol/L��c��OH-��=10y mol/L��x��y�Ĺ�ϵ��ͼ��ʾ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
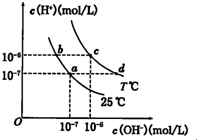 �ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH+������ͼ��ʾ�Ĺ�ϵ������˵���в���ȷ���ǣ�������
�ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH+������ͼ��ʾ�Ĺ�ϵ������˵���в���ȷ���ǣ�������| A��ͼ��T��25 | B��b����Һc��H+��һ����a��� | C��c���Ӧ����Һ�п��ܴ�������Al3+��Cl- | D��d���Ӧ����Һ�ʼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
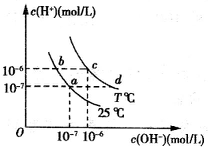 �ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-���Ĺ�ϵ��ͼ��ʾ�������й�˵������ȷ���ǣ�������
�ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-���Ĺ�ϵ��ͼ��ʾ�������й�˵������ȷ���ǣ�������| A������a�㵽c�㣬�ɲ�����ˮ�м�����ķ��� | B��b���Ӧ�Ĵ�������ˮ�����c��H+��=10-6mol/L | C��c���Ӧ��Һ��Kw����d���Ӧ��Һ��Kw | D��T��ʱ��0.05 mol?L-1��Ba��OH��2��Һ��pH=11 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
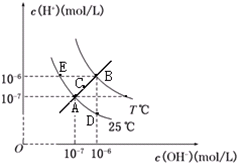 �ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-����ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
�ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-����ϵ��ͼ��ʾ������˵������ȷ���ǣ�������| A��ͼ�����Kw��Ĺ�ϵ��B��C��A=D=E | B��E���Ӧ��ˮ��Һ�У�������NH4+��Ba2+��Cl-��I-����ͬʱ���� | C��������B��ʱ����pH=2��������Һ��pH=10��KOH��Һ�������ϣ�������Һ������ | D����0.1 mol/L��NaHA��Һˮ��Һ��c��H+����c��OH-����ϵ��ͼD����ʾ������Һ���У�c��HA-����c��OH-����c��A2-����c��H2A�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com