
(08���ݰ����ʼ�)���ʵ�ת����ϵ����ͼ��ʾ���еķ�Ӧ������ˮ��Һ�н��У�������AΪ������������ֵ���ֱ�ӻ��ϵõ�����Ϊ�������ʣ�GΪ�ᣬ����G��Ũ��Һ�з����ۻ����еķ�Ӧ�з�Ӧ���������δȫ����������Ӧ����Ҳδע����
 |
��1����AΪ���Ṥҵ����Ҫԭ�ϣ�C��ʹƷ����ɫ��D��ˮ��Һ�м���HNO3�ữ��AgNO3��Һ�а�ɫ�������ɣ�A���Һ�һ����ͬ��Ԫ�ء���
��A�ĵĻ�ѧʽΪ ����ҵ�Ϸ�Ӧ���� �н��У����豸���ƣ�����ҵ�Ϸ�Ӧ����������E���Լ��� ��
�����ռ���Һ����F��Һ������������ʱ�۲쵽������Ϊ
���з�����������ԭ��Ӧ�Ļ�ѧ����ʽΪ
��2������Ϊ����ɫ���壬D��F����Һ���ʼ��ԣ��������������ֱ�պȡA��G��Ũ��Һ��ʹ���ǽӽ����д����������ɡ���
��д��B�ͼ�Ӧ�Ļ�ѧ����ʽ_______________________��
��д��D��Һ���ҷ�Ӧ�����ӷ���ʽ
��E��G�Ļ�ѧ����ʽ�� ________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(08���ݰ����ʼ�)����ͼ��ʾ��װ���У���ƿ��ʢ�п��������ƿ��ʢ����������ʱ���ܷ�����Ȫ��ѡ�� �� �� ��
A�������ϡ����
B��NaOH�����Ũ��ˮ
C��Na2O2��NaOH��Һ
D��Cu��Ũ����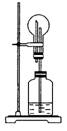
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(08���ݰ����ʼ�)X��Y��Z��WΪ��ԭ��������С�������е����ֶ�����Ԫ�ء���֪��X�ɷֱ���Y��W�γ�X2Y��X2Y2��XW�ȹ��ۻ����
��Z�ɷֱ���Y��W�γ�Z2Y��Z2Y2��ZW�����ӻ����
��ش�
��1��Z2Y�Ļ�ѧʽ�� ��X2Y2�ĵ���ʽ ��Z2Y2��X2Y��Ӧ�Ļ�ѧ����ʽ�� .
��2������ͼ��ʾװ�ã�����������ʢ�����з�̪��
ZW�ı�����Һ��C(I)��C(II)Ϊ���ʯī�缫��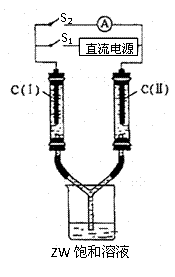
��ͨS1��C(I)������Һ��죬������������
����ۼ�����ʱC(I)Ϊ �����缫��ӦʽΪ
��
һ��ʱ�������������Һ��δ����缫������
��S1����ͨS2��������ָ�뷢��ƫת����ʱ
C(II)Ϊ ���� �缫��ӦʽΪ
��
��3��ͭм����ϡ���������Ӧ������ϡ�����м���
X2Y2��ͭм�����ܽ⣬�÷�Ӧ�����ӷ���ʽ�ǣ�
�����÷���ʽͬʱΪһ��ԭ���װ�õ���
��Ӧʽ�����ԭ��ظ�����ӦʽΪ ��������ӦʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(08���ݰ����ʼ�)X��Y��Z��M���ֽ�������֪X���Դ�Y������Һ���û���Y��X��Z��ϡ�����й���ԭ���ʱ��ZΪ��������ʯī�缫��⺬Y��Z�����ӵ���Һʱ������������Y��M������������ǿ��Y�����ӣ�����Щ�����Ļ����ǿ������˳���� �� ��
A��X��Y��Z��M B��X��Z��M ��Y
C��M�� Z�� X��Y D��X��Z��Y��M
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(08���ݰ����ʼ�)������ʾ�����������ڴ����а���ȼ�գ���������ijУ��ѧС��ѧ���������װ�ã�ͼ�����еȼг�װ������ȥ�����а����������ڲ�ͬ�����·�Ӧ��ʵ�顣
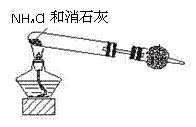
��
��1������װ�â���ȡ����������İ��������Թܷ����ķ�Ӧ��ѧ����ʽ�� ���������ʢװ�������� ��
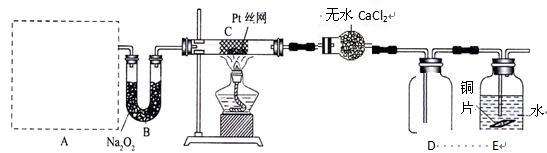
��2������װ�â����ʵ�飬A�������巢��װ�ã�A������ʵ��ҩƷ������������ѡȡ��(a)Na2CO3 (b)NaHCO3 (c)NH4HCO3 (d)NH4Cl (e)Ca(OH)2 (f)Ũ��ˮ�� Ҫ�����װ��C��Pt˿�����������е������ǰ����봿�������������ȷ�����Ӧ��
�ش��������⣺
����A����ȡ����ʱֻ��һ��ҩƷ����ҩƷΪ ���ѡҩƷ����ţ���
��Bװ�õ�����Ϊ ��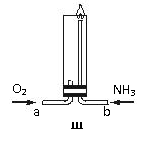
��д��C�з�����Ӧ�Ļ�ѧ����ʽ
�ܷ�Ӧ������װ��D�������Ϊ����ɫ����װ��E��CuƬ�����ķ�Ӧ���ӷ���ʽΪ ��
��3���������Ĵ�����װ�â�����İ����ֱ��a��b���ܽ�����ͨ�뵽װ�â���ͼ���У�����b���϶˵�ȼ��������������ȼ�ղ���������Ⱦ���ʣ���������ͨ����Ⱥ�˳���� ������������ �����ڰ���ȼ�յĻ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(08���ݰ����ʼ�)���л�������������������Ҫ��ѧ��Ӧ�����漰������ԭ��Ӧ���� �� ��
A��������ͨ���� B���Ӵ���������
C����ҵұ���� D����ҵ�ϳɰ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com