
��10�֣���֪��
���� ��R��R���ɱ�ʾ����������ţ�
����A�ɷ�������ת�������������ʾ�Ϊ�л����������������ȥ����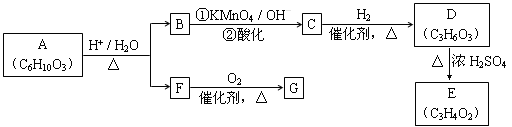
��ش�
��1��F�������ܶ�����ͬ������H2�ܶȵ�31�����ҷ�����������֪1 mol F���������������ò���H2 22.4 L����״��������F�ķ���ʽ�� �������� ��
��2��G��F����Է�������֮��Ϊ4����G���е������� ������ĸ����
a������������Һ��Ӧ���� b���������ᷢ��������Ӧ
c���������������ӳɷ�Ӧ ��d��1 mol G������2 mol����Cu(OH)2������Ӧ
��3��D����NaHCO3��Һ������Ӧ����������D���Է�Ӧ�õ�������Ԫ����������E��ʹ������Ȼ�̼��Һ��ɫ����D��E�Ļ�ѧ����ʽ�� ��
�÷�Ӧ������ ��Ӧ��
��4��H��B��Ϊͬ���칹�壬��������������B��ͬ����H�Ľṹ��ʽ�����ǣ�
�� ��
��5��Aת��ΪB��F�Ļ�ѧ����ʽ�� ��
��10�֣���1��C2H6O2 ��1�֣� ���Ҷ��� ��1�֣���
��2��a c ��1�֣� ŨH2SO4
��3�� CH3-CH-COOH ------�� CH2="CH-COOH" + H2O ��2�֣� �� ��ȥ ��1�֣�
�O ����
OH
��4��CH2=CH-CH2COOH ��1�֣���CH3-CH="CH-COOH " ��1�֣�
H+
��5�� CH3-C-COOCH2CH2OH + H2O -----�� CH3-C-COOH + CH2-CH2 ��2�֣�
�� ���� �� �O �O
CH2 CH2 OH OH
����


 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
T��X��Y��Z��R��WΪ���ڱ�ǰ������Ԫ�أ�ԭ���������ε�������֪��
��Wԭ���������1�����ӣ�����ds��Ԫ�أ�����ľ�Ϊ����������Ԫ�أ�
��Tԭ�����������������������ֱ�����ԭ��������ȣ�
��X�Ļ�̬ԭ���е���ռ������������ͬ��ԭ�ӹ������ÿ�ֹ���еĵ�������ͬ��
��Z�Ļ�̬ԭ�Ӽ۵����Ų�Ϊns2npn+2��
��R�ĵ��ʳ��³�ѹ�������壬���̬ԭ�ӵ�M������1��δ�ɶԵ�p���ӣ�
(1)X��Y��Z����Ԫ�صĵ�һ�������ɴ�С��˳������ ������__________ ___����Ԫ�ط��ű�ʾ����
(2)Y���⻯����Ӽ����γ������R���⻯����Ӽ䲻���γ������ԭ���������������� ����������������
(3)W�Ļ�̬ԭ�ӵļ۲�����Ų�ʽΪ��������������������������������Ԫ����Ԫ��Y ��T���γɵ�[W(YT3)4]2+�����У����еĻ�ѧ������������������
a�����Ӽ���������b�����Լ�����������c���Ǽ��Լ�����������d����λ��
������ṹʽ_____________
(4) T��X��Z����Ԫ����ɵ�һ�ֻ�����M����װ�����г������к����壬���ķ���ʽΪXT2Z�����ӿռ乹��Ϊƽ�������Σ���÷���������ԭ�Ӳ�ȡ_____���������������������ӻ���1molM�����ЦҼ��ͦм��ĸ�����Ϊ�������������������������� ��
(5) T��Z�����γɸ�����Ϊ1:1�ķ��ӣ���������ԭ�Ӳ�ȡ�����������ӻ���ͨ������£��÷�����ˮ����Ȼ��ܵ���Ҫԭ������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012������ʡ������ѧ������һ���¿���ѧ�Ծ� ���ͣ������
T��X��Y��Z��R��WΪ���ڱ�ǰ������Ԫ�أ�ԭ���������ε�������֪��
��Wԭ���������1�����ӣ�����ds��Ԫ�أ�����ľ�Ϊ����������Ԫ�أ�
��Tԭ�����������������������ֱ�����ԭ��������ȣ�
��X�Ļ�̬ԭ���е���ռ������������ͬ��ԭ�ӹ������ÿ�ֹ���еĵ�������ͬ��
��Z�Ļ�̬ԭ�Ӽ۵����Ų�Ϊns2npn+2��
��R�ĵ��ʳ��³�ѹ�������壬���̬ԭ�ӵ�M������1��δ�ɶԵ�p���ӣ�
(1)X��Y��Z����Ԫ�صĵ�һ�������ɴ�С��˳������ ������__________ ___����Ԫ�ط��ű�ʾ����
(2)Y���⻯����Ӽ����γ������R���⻯����Ӽ䲻���γ������ԭ���������������� ����������������
(3)W�Ļ�̬ԭ�ӵļ۲�����Ų�ʽΪ���������������������������� ����Ԫ����Ԫ��Y ��T���γɵ�[W(YT3)4]2+�����У����еĻ�ѧ���������������� ��
a�����Ӽ���������b�����Լ�����������c���Ǽ��Լ�����������d����λ��
������ṹʽ_____________
(4) T��X��Z����Ԫ����ɵ�һ�ֻ�����M����װ�����г������к����壬���ķ���ʽΪXT2Z�����ӿռ乹��Ϊƽ�������Σ���÷���������ԭ�Ӳ�ȡ_____�������������������� �ӻ���1molM�����ЦҼ��ͦм��ĸ�����Ϊ�������������������������� ��
(5) T��Z�����γɸ�����Ϊ1:1�ķ��ӣ���������ԭ�Ӳ�ȡ�����������ӻ���ͨ������£��÷�����ˮ����Ȼ��ܵ���Ҫԭ������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ������һ���¿���ѧ�Ծ� ���ͣ������
T��X��Y��Z��R��WΪ���ڱ�ǰ������Ԫ�أ�ԭ���������ε�������֪��
��Wԭ���������1�����ӣ�����ds��Ԫ�أ�����ľ�Ϊ����������Ԫ�أ�
��Tԭ�����������������������ֱ�����ԭ��������ȣ�
��X�Ļ�̬ԭ���е���ռ������������ͬ��ԭ�ӹ������ÿ�ֹ���еĵ�������ͬ��
��Z�Ļ�̬ԭ�Ӽ۵����Ų�Ϊns2npn+2��
��R�ĵ��ʳ��³�ѹ�������壬���̬ԭ�ӵ�M������1��δ�ɶԵ�p���ӣ�
(1)X��Y��Z����Ԫ�صĵ�һ�������ɴ�С��˳������ ������__________ ___����Ԫ�ط��ű�ʾ����
(2)Y���⻯����Ӽ����γ������R���⻯����Ӽ䲻���γ������ԭ���������������� ����������������
(3)W�Ļ�̬ԭ�ӵļ۲�����Ų�ʽΪ���������������������������� ����Ԫ����Ԫ��Y ��T���γɵ�[W(YT3)4]2+�����У����еĻ�ѧ���������������� ��
a�����Ӽ���������b�����Լ�����������c���Ǽ��Լ�����������d����λ��
������ṹʽ_____________
(4) T��X��Z����Ԫ����ɵ�һ�ֻ�����M����װ�����г������к����壬���ķ���ʽΪXT2Z�����ӿռ乹��Ϊƽ�������Σ���÷���������ԭ�Ӳ�ȡ_____�������������������� �ӻ���1molM�����ЦҼ��ͦм��ĸ�����Ϊ�������������������������� ��
(5) T��Z�����γɸ�����Ϊ1:1�ķ��ӣ���������ԭ�Ӳ�ȡ�����������ӻ���ͨ������£��÷�����ˮ����Ȼ��ܵ���Ҫԭ������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪������A����Է�������Ϊ182.5���������ɿ��Ա�ʾΪCxHyOCl���й�ת����ϵ��ͼ��ʾ��

�� C��һ�������¿ɷ�����Ӧ������һ�ָ߷��ӻ�����E��E�Ľṹ��ʽΪ
 ������R��R��������
������R��R��������
��ش��������⣺
��1��A�к��еĺ��������ŵ����������������������������� ��
�۵ķ�Ӧ����Ϊ������������������������������ ��
��2����֪��������R���л���R�DOH����50%����A�Ļ�ѧʽΪ�������������� ��
��3����֪E��R�������ȡ�����ʶ�λ����E�Ľṹ��ʽΪ�������������������� ��
��4��д����Ӧ�ٵĻ�ѧ����ʽ���������������������������������������������� ��
��5��D��Ũ���ᡢ���ȵ������·�Ӧ����F(C11H12O2)��F��һ�������¿��Է����Ӿ۷�Ӧ��д���üӾ۷�Ӧ�Ļ�ѧ����ʽ�������������������������������������� ��
��6����B�Ķ���ͬ�������У�д�����ӽṹ�к��� �������� ����������ͬ���칹��Ľṹ��ʽ�� ���������������������� ��
�������� ����������ͬ���칹��Ľṹ��ʽ�� ���������������������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л���A�������ΪC4H9Br��A����һ��֧������һ��������A�ɷ�������ת����
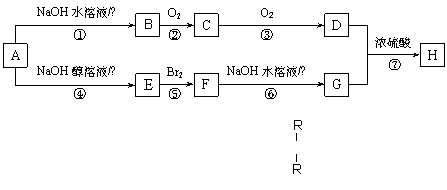 | |||
| |||
��֪�������ǻ���̼ԭ����û����ԭ�ӵĴ����� R��C��OH�����ܷ�����������Ӧ��
��1��A�Ľṹ��ʽΪ������������������ ��F�Ľṹ��ʽΪ���������������� ��
��2������ת��������ȡ����Ӧ������������ ������ţ���
��3����Ӧ�ܵĻ�ѧ����ʽΪ������������������������������������������������ ��C����������Ӧ�Ļ�ѧ����ʽΪ������������������������������������������������ ��
E�����Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ������������������������������������������������ ��
��4����Ӧ���У�D��G���ʵ���֮��Ϊ2��1����÷�Ӧ�Ļ�ѧ����ʽΪ��
����������������������������������������������������������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com