
 H2SO3
H2SO3 H2SO3
H2SO3
| ||
| ||
 H2SO3��
H2SO3�� H2SO3�� ��SO2+CaO=CaSO3����SO2+2NaOH=Na2SO3+H2O��
H2SO3�� ��SO2+CaO=CaSO3����SO2+2NaOH=Na2SO3+H2O��
| ||
| ||


 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д� һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��)�ǽ������ʼ��仯�����������Ϳ����ж�����ҪӦ�á�
(1) ��������������й©�¹ʣ����д�ʩ����ȷ����_________��
a������ͨ����ز��ţ�Ѹ�ٳ����¹��ֳ�
b����պ�з���ˮ��ë����ס�ڱ����������ɢ
c����պ��NaOH��Һ��ë����ס�ڱ�����˳����ɢ
(2) �¹ʷ�������NaOHϡ��Һ����й©����������Ӧ�����ӷ���ʽ��________��
(3) ��Na2SO3��Na2S�Ļ����Һ�м���ϡ���ᣬ��Һ�л������������ɫ��������÷�Ӧ���������ͻ�ԭ�������ʵ���֮����__________
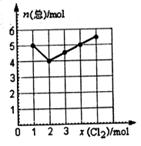
(4) Cl2��NO2��һ�������·������Ϸ�Ӧ������һ�����壬ʵ��������ͼ��ͼ�к������Ǽ���C12�����ʵ������������Ƿ�Ӧ���������ʵ����ܺ͡���֪��ȡC12��NO2�����ʵ����ܺ�Ϊ6 mol����������Ļ�ѧʽ��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�콭��ʦ���и�����ѧ�����п��Ի�ѧ�� ���ͣ������
(8��)�ǽ������ʼ��仯�����������Ϳ����ж�����ҪӦ�á�
(1) ��������������й©�¹ʣ����д�ʩ����ȷ����_________��
a������ͨ����ز��ţ�Ѹ�ٳ����¹��ֳ�
b����պ�з���ˮ��ë����ס�ڱ����������ɢ
c����պ��NaOH��Һ��ë����ס�ڱ�����˳����ɢ
(2) �¹ʷ�������NaOHϡ��Һ����й©����������Ӧ�����ӷ���ʽ��________��
(3) ��Na2SO3��Na2S�Ļ����Һ�м���ϡ���ᣬ��Һ�л������������ɫ��������÷�Ӧ���������ͻ�ԭ�������ʵ���֮����__________
(4) Cl2��NO2��һ�������·������Ϸ�Ӧ������һ�����壬ʵ��������ͼ��ͼ�к������Ǽ���C12�����ʵ������������Ƿ�Ӧ���������ʵ����ܺ͡���֪��ȡC12��NO2�����ʵ����ܺ�Ϊ6 mol����������Ļ�ѧʽ��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʦ���и�����ѧ�����п��Ի�ѧ�� ���ͣ������
(8��)�ǽ������ʼ��仯�����������Ϳ����ж�����ҪӦ�á�
(1) ��������������й©�¹ʣ����д�ʩ����ȷ����_________��
a������ͨ����ز��ţ�Ѹ�ٳ����¹��ֳ�
b����պ�з���ˮ��ë����ס�ڱ����������ɢ
c����պ��NaOH��Һ��ë����ס�ڱ�����˳����ɢ
(2) �¹ʷ�������NaOHϡ��Һ����й©����������Ӧ�����ӷ���ʽ��________��
(3) ��Na2SO3��Na2S�Ļ����Һ�м���ϡ���ᣬ��Һ�л������������ɫ��������÷�Ӧ���������ͻ�ԭ�������ʵ���֮����__________
(4) Cl2��NO2��һ�������·������Ϸ�Ӧ������һ�����壬ʵ��������ͼ��ͼ�к������Ǽ���C12�����ʵ������������Ƿ�Ӧ���������ʵ����ܺ͡���֪��ȡC12 ��NO2�����ʵ����ܺ�Ϊ6 mol����������Ļ�ѧʽ��__________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ������
 ������δ�ɼ��۵��ӣ����ߴ���һ����ѧ����
������δ�ɼ��۵��ӣ����ߴ���һ����ѧ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ǽ������ʼ��仯�����������Ϳ����ж�����ҪӦ�á�
(1) ��������������й©�¹ʣ����д�ʩ����ȷ����_________��
a������ͨ����ز��ţ�Ѹ�ٳ����¹��ֳ�
b����պ�з���ˮ��ë����ס�ڱ����������ɢ
c����պ��NaOH��Һ��ë����ס�ڱ�����˳����ɢ
(2) �¹ʷ�������NaOHϡ��Һ����й©����������Ӧ�����ӷ���ʽ��________��
(3) ��Na2SO3��Na2S�Ļ����Һ�м���ϡ���ᣬ��Һ�л������������ɫ��������÷�Ӧ���������ͻ�ԭ�������ʵ���֮����__________
(4) Cl2��NO2��һ�������·������Ϸ�Ӧ������һ�����壬ʵ��������ͼ��ͼ�к������Ǽ���C12�����ʵ������������Ƿ�Ӧ���������ʵ����ܺ͡���֪��ȡC12 ��NO2�����ʵ����ܺ�Ϊ6 mol����������Ļ�ѧʽ��__________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com