
 CH3COO-+H+��
CH3COO-+H+������ ��1���Ҵ��������������ᣬ���������ζ��
��2��ˮ������Ҫ�ɷ�Ϊ̼��ƣ�������̼��Ʒ�Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2CH3COOH=��CH3COO��2Ca+H2O+CO2�������ݻ�ѧ����ʽд����ȷ�����ӷ���ʽ��
��3�����ݴ�����һԪ���ᣬ���ֵ��룻
��4������Ҫ�ɷ�ΪCH3COOH���Ƶ���Ҫ�ɷ�ΪCH3CH2OH�����߿��Է���������Ӧ����CH3COOCH2CH3��
��� �⣺��1���Ҵ��������������ᣬ��Ӧ�ķ���ʽΪ��CH3CH2OH+O2��CH3COOH+H2O��
�ʴ�Ϊ��CH3CH2OH+O2��CH3COOH+H2O��
��2��������̼��Ʒ�Ӧ���ɴ���ƺ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽ�ɱ�ʾΪ��CaCO3+2CH3COOH=��CH3COO��2Ca+H2O+CO2������Ӧ�����ӷ���ʽΪ��CaCO3+2CH3COOH=Ca2++2CH3COO-+H2O+CO2����
�ʴ�Ϊ��CaCO3+2CH3COOH=Ca2++2CH3COO-+H2O+CO2����
��3��������һԪ���ᣬ���ֵ��룬���뷽��ʽΪ��CH3COOH CH3COO-+H+��
CH3COO-+H+��
�ʴ�Ϊ��CH3COOH CH3COO-+H+��
CH3COO-+H+��
��4������Ҫ�ɷ�ΪCH3COOH���Ƶ���Ҫ�ɷ�ΪCH3CH2OH�����߿��Է���������Ӧ����CH3COOCH2CH3����ѧ����ʽΪ��CH3COOH+CH3CH2OH?CH3COOCH2CH3+H2O��
�ʴ�Ϊ��CH3COOH+CH3CH2OH?CH3COOCH2CH3+H2O��������Ӧ��
���� ���⿼���Ҵ�����������ʣ��漰�Ҵ��Ĵ�������Ӧ�Լ������ᷢ����������Ӧ��֪ʶ�������л���Ӧ�Ķ��ѻ�ѧ�����γɻ�ѧ�����������Ӧ�������ǽ���Ĺؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������Ƶĵ���ʽ��Na${\;}_{•}^{•}$$\underset{\stackrel{••}{O}}{••}$${\;}_{•}^{•}$$\underset{\stackrel{••}{O}}{••}$${\;}_{•}^{•}$Na | |
| B�� | ������Ϊ35��������Ϊ45����ԭ�ӣ�${\;}_{35}^{80}$Br | |
| C�� | �����ӵĽṹʾ��ͼ��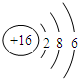 | |
| D�� | ����ױ��Ľṹ��ʽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
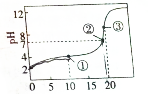 25��ʱ����20mL0.2mo•lL-1HR��Һ������0.2mol•L-1NaOH��Һ���õ���ͼ�ĵζ����ߣ���������Ũ�ȵĹ�ϵʽ������ǣ�������
25��ʱ����20mL0.2mo•lL-1HR��Һ������0.2mol•L-1NaOH��Һ���õ���ͼ�ĵζ����ߣ���������Ũ�ȵĹ�ϵʽ������ǣ�������| A�� | �����Һ�У�c��HR��+2c��H+��=c��R-��+2c��OH-�� | |
| B�� | �����Һ�У�c��Na+��=c��HR��+c��R-�� | |
| C�� | �����Һ�У�c��Na+����c��R-������OH-����c��H+�� | |
| D�� | �ζ������п��ܻ���֣�c��HR����c��R-������H+����c��Na+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 950mL��76g | B�� | 500mL��80g | C�� | 1000mL��80g | D�� | 1000mL��76g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
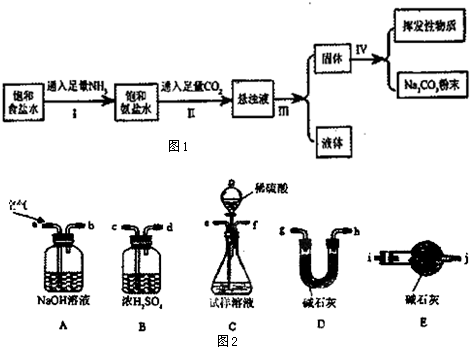
| ���� | NH4Cl | NaHCO3 | Na2CO3 | NaCl |
| �ܽ��/g | 37.2 | 9.6 | 21.5 | 36.0 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com