
ĻĀĮŠĖµ·ØÕżČ·µÄÓŠ(””””)
¢ŁSiO2æÉÓėHF·“Ó¦£¬Ņņ¶ųĒā·śĖį²»Äܱ£“ęŌŚ²£Į§ĘæÖŠ
¢ŚŹµŃéŹŅÖĘČ”ĀČĘųŹ±£¬ĪŖĮĖ·ĄÖ¹»·¾³ĪŪČ¾£¬¶ąÓąµÄĀČĘųæÉŅŌÓĆĒāŃõ»ÆøĘČÜŅŗĪüŹÕ
¢ŪĻņ·ŠĖ®ÖŠÖšµĪ¼ÓČėÉŁĮ汄ŗĶFeCl3ČÜŅŗ£¬æÉÖʵĆFe(OH)3½ŗĢå
¢ÜNa2O”¢Na2O2×é³ÉŌŖĖŲĻąĶ¬£¬ŃōĄė×ÓÓėŅõĄė×ÓøöŹż±ČŅ²ĻąĶ¬
¢Ż³żČ„HC lĘųĢåÖŠµÄCl2£¬æɽ«ĘųĢåĶØČė±„ŗĶŹ³ŃĪĖ®ÖŠ
lĘųĢåÖŠµÄCl2£¬æɽ«ĘųĢåĶØČė±„ŗĶŹ³ŃĪĖ®ÖŠ
¢ŽĻņĀČĖ®ÖŠ¼ÓCaCO3·ŪÄ©£¬æÉĢįøßČÜŅŗÖŠHClOµÄÅضČ
¢ßSiO2ÄÜÓėNaOHČÜŅŗ”¢HFČÜŅŗ·“Ó¦£¬ĖłŅŌSiO2ŹĒĮ½ŠŌŃõ»ÆĪļ
A. 3øö B. 4øö C. 5øö D. 6øö
 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğŗŚĮś½Ź”ĵµ¤½ŹŠø߶žÉĻѧʌæŖѧ¼ģ²ā»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
Įņ“śĮņĖįÄĘČÜŅŗÓėĻ”ĮņĖį·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
Na2S2O3 + H2SO4 = Na2SO4 + SO2 + S”ż + H2O
ĻĀĮŠø÷×鏵ŃéÖŠ×īĻČ³öĻÖ»ė×ĒµÄŹĒ
ŹµŃé | ·“Ó¦ĪĀ¶Č/”ę | Na2S2O3ČÜŅŗ | Ļ”H2SO4 | H2O | ||
V/mL | c/(mol”¤L-1) | V/mL | c/(mol”¤) | V/mL | ||
A | 25 | 5 | 0.1 | 10 | 0.1 | 5 |
B | 25 | 5 | 0.2 | 5 | 0.2 | 10 |
C | 35 | 5 | 0.1 | 10 | 0.1 | 5 |
D | 35 | 5 | 0.2 | 5 | 0.2 | 10 |
A. A B. B C. C D. D
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğ°²»ÕŹ”Āķ°°É½ŹŠø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
(1)ÓĆCH4“߻ƻ¹ŌNOxæÉŅŌĻū³żµŖŃõ»ÆĪļµÄĪŪČ¾”£ĄżČē£ŗ
CH4(g)£«4NO2(g)===4NO(g)£«CO2(g)£«2H2O(g)”” ¦¤H£½£574 kJ”¤mol£1
CH4(g)£«4NO(g)===2N2(g)£«CO2(g)£«2H2O(g) ¦¤H£½£1 160 kJ”¤mol£1
ČōÓƱź×¼×“æöĻĀ4.48 L CH4»¹ŌNO2Éś³ÉN2£¬·“Ó¦ÖŠ×ŖŅʵĵē×Ó×ÜŹżĪŖ________ (°¢·ü¼ÓµĀĀŽ³£ŹżÓĆNA±ķŹ¾)£¬·Å³öµÄČČĮæĪŖ________kJ”£
(2)ŅŃÖŖ£ŗC3H8(g) == CH4(g)£«HC”ŌCH(g)£«H2(g)””¦¤H1£½£«156.6 kJ”¤mol£1
CH3CH=CH2(g) == CH4(g)£«HC”ŌCH(g) ¦¤H2£½£«32.4 kJ”¤mol£1
ŌņĻąĶ¬Ģõ¼žĻĀ£¬·“Ó¦C3H8(g) === CH3CH=CH2(g)£«H2(g)µÄ¦¤H£½______kJ”¤mol£1”£
(3)¼×ĶéŌŚøßĪĀĻĀÓėĖ®ÕōĘų·“Ó¦µÄ·½³ĢŹ½ĪŖCH4(g)£«H2O(g)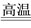 CO(g)£«3H2(g)”£²æ·ÖĪļÖŹµÄČ¼ÉÕČČŹż¾ŻČēĻĀ±ķ£ŗŅŃÖŖ1 mol H2O(g)×Ŗ±äĪŖ1 mol H2O(l)Ź±·Å³ö44.0 kJČČĮ攣Š“³öCH4ŗĶH2OŌŚøßĪĀĻĀ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½£ŗ _______________________________”£
CO(g)£«3H2(g)”£²æ·ÖĪļÖŹµÄČ¼ÉÕČČŹż¾ŻČēĻĀ±ķ£ŗŅŃÖŖ1 mol H2O(g)×Ŗ±äĪŖ1 mol H2O(l)Ź±·Å³ö44.0 kJČČĮ攣Š“³öCH4ŗĶH2OŌŚøßĪĀĻĀ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½£ŗ _______________________________”£
ĪļÖŹ | Č¼ÉÕČČ(kJ”¤mol£1) |
H2(g) | £285.8 |
CO(g) | £283.0 |
CH4(g) | £890.3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğ½Ī÷Ź”ÄĻ²żŹŠøßŅ»ÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ¼ĘĖćĢā
ŌŚMgCl2ŗĶAlCl3µÄ»ģŗĻČÜŅŗÖŠ£¬ÖšµĪ¼ÓČėNaOHČÜŅŗÖ±ÖĮ¹żĮ攣¾²ā¶Ø£¬¼ÓČėµÄNaOHµÄĪļÖŹµÄĮæ(mol)ŗĶĖłµĆ³Įø”µÄĪļÖŹµÄĮæ(mol)µÄ¹ŲĻµČēÓŅĶ¼ĖłŹ¾”£Ōņ£ŗ
£Ø1£©Š“³ö“ś±ķø÷Ļ߶Ī·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
OD¶Ī______________________________£¬
DC¶Ī______________________________”£
£Ø2£©ŌČÜŅŗÖŠMg2£«”¢Al3£«ĪļÖŹµÄĮæÅضČÖ®±ČĪŖ___________”£
£Ø3£©Ķ¼ÖŠCµć±ķŹ¾µ±¼ÓČė_______mol NaOHŹ±,Al3£«ŅŃ¾×Ŗ»Æ³É____________”£
£Ø4£©Ķ¼ÖŠĻ߶ĪOA”ĆAB£½__________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğ½Ī÷Ź”ÄĻ²żŹŠøßŅ»ÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
½«Ä³Š©»ÆѧÖŖŹ¶ÓĆĶ¼Ļń±ķŹ¾£¬æÉŅŌŹÕµ½Ö±¹Ū”¢¼ņĆ÷µÄŠ§¹ū”£ĻĀĮŠĶ¼ĻóĖł±ķŹ¾µÄ»ÆѧÖŖŹ¶ÖŠ£¬Ć÷ĻŌ²»ÕżČ·µÄŹĒ£Ø £©
A. A B. B C. C D. D
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğ½Ī÷Ź”ÄĻ²żŹŠøßŅ»ÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
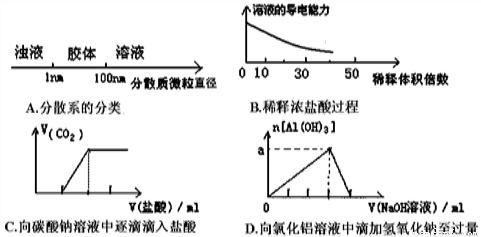
»ÆѧÖŖŹ¶ŌŚÉś²śŗĶÉś»īÖŠÓŠ×ÅÖŲŅŖµÄÓ¦ÓĆ”£ĻĀĮŠĖµ·ØÖŠÕżČ·µÄŹĒ£Ø £©
¢ŁĀĮÖĘ²Ķ¾ß²»ŅĖÓĆÕōÖó»ņ³¤Ź±¼ä“ę·ÅĖįŠŌ”¢¼īŠŌ»ņĻĢµÄŹ³Īļ
¢Ś·¢½Ķ·ŪÖŠÖ÷ŅŖŗ¬ÓŠĢ¼ĖįĒāÄĘ£¬ÄÜŹ¹±ŗÖĘ³öµÄøāµćŹčĖɶąæ×
¢Ū“æ¾»µÄ¶žŃõ»Æ¹čŹĒĻÖ“ś¹āѧ¼°¹āĻĖÖĘĘ·µÄ»ł±¾ŌĮĻ
¢ÜNa2O2¼ČæÉ×÷ŗōĪüĆę¾ßÖŠO2µÄĄ“Ō“£¬ÓÖæÉĘÆ°×ÖÆĪļ”¢ĀóøĖ”¢ÓšĆ«µČ
¢ŻĢ¼ĖįÄĘŌŚŅ½ĮĘÉĻŹĒÖĪĮĘĪøĖį¹ż¶ąµÄŅ»ÖÖŅ©¼Į
¢ŽĆ÷·Æ³£×÷ĪŖĻū¶¾¼Į
¢ßFe2O3£Ø×÷»ĘÉ«ÓĶĘįŗĶĶæĮĻ£©
A. ¢Ł¢Ś¢Ū¢Ü B. ¢Ł¢Ś¢Ū¢Ż¢ß C. ¢Ł¢Ś¢Ü¢Ž¢ß D. ¢Ł¢Ś¢Ū¢Ü¢Ż¢Ž
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğŗŚĮś½Ź”ĵµ¤½ŹŠøßŅ»ĻĀѧʌæŖѧ¼ģ²ā»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©ŹµŃéŹŅÉŁĮæµÄ½šŹōÄʱ£“ęŌŚ___________ÖŠ£¬Č”ÓĆŹ±ÓƵ½µÄŅĒĘ÷ŗĶÓĆĘ·ÓŠŠ”µ¶”¢²£Į§Ę¬”¢ĀĖÖ½ŗĶ____________, Ź£ÓąµÄÄĘÓ¦_______________£»½«Ņ»Š”æéÄĘĶ¶Čėµ½ĮņĖįĶČÜŅŗÖŠ£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ______________________________________£¬¹Ū²ģµ½µÄĻÖĻóĪŖ_____________(ĢīŠ“±źŗÅ)”£
a£®ÄĘø”ŌŚŅŗĆęÉĻĖÄ“¦ÓĪ¶Æ b£®ÄĘČŚ³ÉĮĖŅ»øöÉĮĮĮµÄŠ”Ēņ
c£®ČÜŅŗÖŠÓŠĄ¶É«³ĮµķÉś³É d£®ČÜŅŗÖŠÓŠŗģÉ«¹ĢĢåĪö³ö
£Ø2£©ŹµŃéŹŅÖĘĀČĘųµÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ_________________________________£»ĀČĘųĪŖÓŠ¶¾ĘųĢ壬ŠčÓĆĒāŃõ»ÆÄĘČÜŅŗ½ųŠŠĪ²Ęų“¦Ąķ£¬ĒėĶź³ÉøĆĄė×Ó·½³ĢŹ½____________£¬ øĆŌĄķ»¹æÉÓĆÓŚ¹¤ŅµÖĘ______________”£
£Ø3£©ĻĀĮŠĪļÖŹ¼ČÄÜÓėŃĪĖį·“Ó¦£¬ÓÖÄÜÓėÉÕ¼īČÜŅŗ·“Ó¦µÄŹĒ________________________________
a. Al b. Mg c. CH3COONH4 d. NaHCO3 e. Al2O3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğŗŚĮś½Ź”ĵµ¤½ŹŠøßŅ»ĻĀѧʌæŖѧ¼ģ²ā»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
100ml 1mol/LµÄAlCl3ČÜŅŗÓė100ml 3.5mol/LµÄNaOHČÜŅŗ»ģŗĻ£¬µĆµ½³ĮµķĪŖ( )
A. 7.8g B. 0g C. 9.1 g D. 3.9g
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗŗžÄĻŹ”³¤É³ŹŠ2016-2017ѧğøßŅ»ÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠĄė×Ó·½³ĢŹ½ÕżČ·µÄŹĒ
A. ½«ĀČĘųČÜÓŚĖ®Öʱø“ĪĀČĖį£ŗCl2+H2OØT2H++Cl-+ClO-
B. Ģ¼ĖįøĘÓėĻ”ŃĪĖį·“Ó¦£ŗCO32-+2H+=H2O+CO2”ü
C. ĮņĖįĀĮŗĶ°±Ė®·“Ó¦£ŗAl3++3OH-£½Al(OH)3”ż
D. NaÓėH2O·“Ó¦£ŗ2Na+2H2O=2Na++2OH-+H2”ü
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com