
���� ��1���������Ϊ��ɫ��Һ�������������Ʒ���������ԭ��Ӧ������ɫ�Ķ��������ӣ��ݴ˽��
��2�����Ը�����ؾ���ǿ�����ԣ�ʵ��ʱӦ����ʽ�ζ��ܣ���ɫNaHSO3��Һ�����ԣ�����ʽ�ζ��ܣ��ζ����̻���Ҫ�ձ�����ƿ����ֽ���ζ��ܼк�����̨��
��3��������ؾ���ǿ�������ܸ�ʴ��ʽ�ζ����е��ܣ�
��4���ζ�����Һ�棬����ƫС���ζ�������Һ�棬����ƫ��c�����⣩=$\frac{c��������V������}{V�����⣩}$��$\frac{5}{2}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��5�����ݵ����غ㽨����ϵʽ��Ce��OH��4��FeSO4��Ȼ����м������Ce��OH��4������������������������
��� �⣺��1��Mn2+��ɫ��MnO4- Ϊ��ɫ��������MnO4-ʹ��ɫ��Һ��Ϊ��ɫ�����Ա�ʵ�鲻��Ҫʹ��ָʾ����
�ʴ�Ϊ������Ҫ����Mn2+��ɫ��MnO4- Ϊ��ɫ��������MnO4-ʹ��ɫ��Һ��Ϊ��ɫ��
��2�����Ը�����ؾ���ǿ�����ԣ�ʵ��ʱӦ����ʽ�ζ��ܣ���ɫNaHSO3��Һ�����ԣ�����ʽ�ζ��ܣ��ζ����̻���Ҫ��ƿʢ�Ŵ���Һ����ֽ�Ա��յ���ɫ�仯���ζ��ܼк�����̨��������Ҫ�õ����ǣ�A��D��E��F��G��H����
�ʴ�Ϊ��A��D��E��F��G��H����
��3��������ؾ���ǿ�������ܸ�ʴ��ʽ�ζ����е��ܣ����Բ����ü�ʽ�ζ���ʢ�Ÿ��������Һ��Ӧ������ʽ�ζ��ܣ�
�ʴ�Ϊ�������KMnO4��Һ�ḯʴ��ʽ�ζ����¶˽��ܣ�
��4���ζ�ǰƽ��KMnO4Һ�棬�̶�Ϊa mL���ζ�����Һ��̶�Ϊb mL������ƫС����b-a��mL��ʵ������KMnO4��Һ����٣�
����ζ�������Һ��̶�Ϊcml����c-a��mL��ʵ�����ĸ���������������c�����⣩=$\frac{c��������V������}{V�����⣩}$��$\frac{5}{2}$��֪����ҺŨ�ȱ�ʵ��Ũ��ƫ��
�ʴ�Ϊ���٣� ��
��5��Ce��OH��4 ��FeSO4
0.0020mol 0.1000mol/L-1��0.020L
����m��Ce��OH��4��=0.0020mol��208g/mol=0.416g����Ʒ��Ce��OH��4����������Ϊ
$\frac{0.416g}{0.5g}$��100%=83.20%��
�ʴ�Ϊ��83.20%��
���� ���⿼���к͵ζ����������㣬Ϊ��Ƶ���㣬������ѧ���ķ����������ʵ�������Ŀ��飬ע������к͵ζ��IJ���ԭ����ʵ�鷽�����Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | BaSO4������ˮ������BaSO4�Ƿǵ���� | |
| B�� | ������ʵĵ�����һ����ǿ������� | |
| C�� | 25��ʱ0.1mol/L��CH3COOH��ҺpH=3��˵��CH3COOHΪ������� | |
| D�� | ij�����ܵ��磬���Ը�����һ���ǵ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��� | ��������ĩ״����mol�� | ���Ũ�ȼ���� | ��Ӧ�¶ȣ��棩 | ||
| A | Al | 0.1 | 0.1mol•L-1 ���� | 10mL | 60 |
| B | Fe | 0.1 | 0.2mol•L-1���� | 10mL | 60 |
| C | Al | 0.1 | 18mol•L-1 ���� | 10mL | 60 |
| D | Mg | 0.1 | 0.2mol•L-1 ���� | 10mL | 60 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
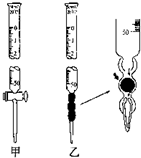 ijѧϰС����0.80mol/L��Ũ�ȵ��ռ���Һ�ⶨδ֪Ũ�ȵ����ᣮ
ijѧϰС����0.80mol/L��Ũ�ȵ��ռ���Һ�ⶨδ֪Ũ�ȵ����ᣮ| ʵ���� | ����HCl��Һ�����/mL | ����NaOH��Һ�����/mL |
| 1 | 20.00 | 22.00 |
| 2 | 20.00 | 22.10 |
| 3 | 20.00 | 21.90 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ���� | NaOH��Һ��Ũ�ȣ�mol•L-1�� | �ζ����ʱ��NaOH��Һ����������mL�� | ��������������mL�� |
| 1 | 0.10 | 22.62 | 20.00 |
| 2 | 0.10 | 22.72 | 20.00 |
| 3 | 0.10 | 22.80 | 20.00 |
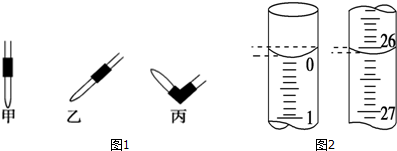
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
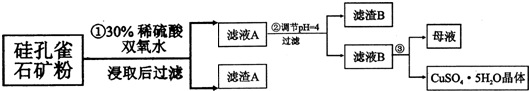
| �������� | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 |
| ��ʼ������pH | 3.3 | 1.5 | 6.5 | 4.2 |
| ������ȫ��pH | 5.2 | 3.7 | 9.7 | 6.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
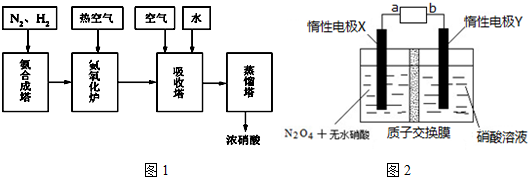
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
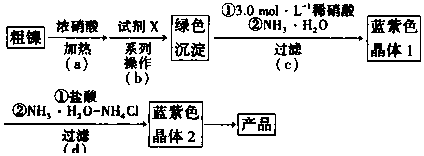
| Fe3+ | Cr3+ | Ni2+ | |
| ��ʼ����pH | 1.5 | 4.3 | 6.9 |
| ��ȫ����pH | 2.8 | 5.6 | 8.9 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com