
(1)�����ƴ�Լ1 mol��L-1����Һ100 mL����������ֻ��������ƽ����Ͳ�����̶ȵ��ձ��������������п�ѡ�õ��Լ���__________(ѡ����ĸ���)��
A.�������ƹ��� B.Ũ����(98��) C.Ũ����(37��) D.Ũ����(63��)
(2)��ͼ��ijѧ��������0.100 mol��L-1��̼������Һ100 mLʱ������һ��ϴ��Һת�Ƶ�����ƿ�ڵIJ�����
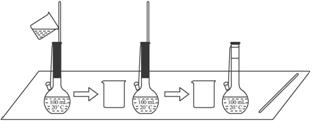
�ò�������������ҺŨ�ȵ�Ӱ����__________(�ƫ�ߡ���ƫ�͡�����Ӱ�족)��ԭ����_______
_____________________________________________________________________��
(3)������(2)����Һʱ������ʹ��������ƽ��ȡ̼���ƣ����������һ����?Ϊʲô?_______
____________________________________________________________________��
(4)������Һ��ȷ��ȡ20.0 mL 0.100 mol��L-1��̼������Һ��ע����ƿ�еμӼ����Լ�2��3�Σ���ʱ��Һ��__________ɫ��Ȼ����ϡ����ע��ྻ������ĵζ����ڣ����µζ�����ϡ����ij�ʼ�̶ȶ��������ſ�ʼ����ƿ�ڵμ����ᡣ����ƿ����Һ��ɫͻ���__________ɫʱ���ﵽ�ζ��յ㣬��ʱ�����������Ϊ21.36 mL������������Ũ��Ϊ__________ mol��L-1��
(1)ABCD
(2)ƫ�ͣ�
�ٲ�������������ƿ�ڣ����ϴ��Һ����
�ڲ�����������ƿ�Ƴ�ʱ�������ڲ������ϵ�ϴ��Һ���ܵ���ƿ��
�۲������Ƴ���ֱ�ӷ��������ϣ���ɲ���ϴ��Һ��ʧ
��մ�۲�����
(3)̼���Ƶļ�����Ϊ
(4)�� �� 0.187
�����������ʱ��Ҫ�ع�ʵ��Ĺ��̣���עʵ���ϸ�ڡ���Na2CO3�ε�NaCl�����ȴ�ʱӦ�Ժ�ɫ��c(HCl)��21.36��10



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢� | B���٢� | C���ڢ� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ȡ����ʱ��ѡ���л���ȡ��������������ȡ�����ܽ�ȱ����ˮ�� | B����Һ����ʱ����Һ©�����²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� | C���������ʱ��Ӧʹ�¶ȼ�ˮ�����������Һ��Һ���� | D������һ�����ʵ���Ũ�ȵ���Һʱ��ϴ���ձ��Ͳ���������Һ����ת������ƿ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com