
����Ŀ��ij��ɫ���ʼ�ֻ������Ԫ�أ�Ϊ̽�����ʼ���ɺ����ʣ���Ʋ��������ʵ�顣������̬�⻯�����ڱ���µ��ܶ�Ϊ 1.518 g��L-1����������ͬԪ�صĻ��ϼ���ͬ��
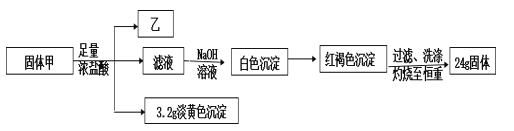
��1�������Ԫ��_____________��
��2��д����������Ũ���ᷴӦ�����ӷ���ʽ______________��
��3���������ڹ��������г��ȼ�պ��ٽ��������ͨ��BaCl2��Һ�����ְ�ɫ������д���ù������ܷ�Ӧ�����ӷ���ʽ_________________ ��
���𰸡�Fe��S Fe3S4 +6H+=3Fe2++S��+H2S�� 2SO2+O2+2H2O+2Ba2+=2BaSO4��+4H+
��������
��������Ũ���ᷴӦ�õ�����Һ�м�������NaOH��Һ��������ɫ��������ɫ������Ϊ���ɫ����Һ�к�Fe2+�����ɫ����ΪFe(OH)3�����ɫFe(OH)3���պ�õ�24g�Ĺ���ΪFe2O3������Fe�غ㣬���к�FeԪ�أ���n(Fe)=2n(Fe2O3)=![]() 2=0.3mol����+Ũ����
2=0.3mol����+Ũ����![]() ��̬�⻯����+��Һ+����ɫ����������ֻ������Ԫ�أ�M(��)=1.518g/L
��̬�⻯����+��Һ+����ɫ����������ֻ������Ԫ�أ�M(��)=1.518g/L![]() 22.4L/mol��34g/mol����ΪH2S������ɫ����ΪS��n(S)=
22.4L/mol��34g/mol����ΪH2S������ɫ����ΪS��n(S)=![]() =0.1mol�����к�SԪ�أ�������Ԫ�صĻ��ϼ�Ϊ-2�ۣ���������ͬԪ�صĻ��ϼ���ͬ��������Ԫ�صĻ��ϼ�Ϊ-2�ۣ�����Ũ����ķ�ӦΪ������ԭ��Ӧ�������FeԪ�ص�ƽ�����ϼ�Ϊx�����ݵ�ʧ�����غ㣬0.3mol
=0.1mol�����к�SԪ�أ�������Ԫ�صĻ��ϼ�Ϊ-2�ۣ���������ͬԪ�صĻ��ϼ���ͬ��������Ԫ�صĻ��ϼ�Ϊ-2�ۣ�����Ũ����ķ�ӦΪ������ԭ��Ӧ�������FeԪ�ص�ƽ�����ϼ�Ϊx�����ݵ�ʧ�����غ㣬0.3mol![]() [x-(+2)]=0.1mol
[x-(+2)]=0.1mol![]() [0-(-2)]�����x=
[0-(-2)]�����x=![]() �������������ϼ۴�����Ϊ0���Ļ�ѧʽΪFe3S4��
�������������ϼ۴�����Ϊ0���Ļ�ѧʽΪFe3S4��
��1��������������֪���Ļ�ѧʽΪFe3S4���ʼ���Fe��S����Ԫ����ɣ�
�������Fe��S��
��2����������Ũ���ᷴӦ�����ӷ���ʽΪ��Fe3S4 +6H+=3Fe2++S��+H2S����
�������Fe3S4 +6H+=3Fe2++S��+H2S����
��3����������H2S���ڹ��������г��ȼ�պ�����SO2����ȼ�պ�Ļ�����壨SO2��O2��ͨ��BaCl2��Һ�����ְ�ɫ����BaSO4�����ӷ���ʽΪ��2SO2+O2+2H2O+2Ba2+=2BaSO4�� +4H+��
�������2SO2+O2+2H2O+2Ba2+=2BaSO4 ��+4H+��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NaClΪ��Ҫ�ɷֵ���ѩ���ḯʴ����������ȸ����豸��ij�о�С��̽��NaCl��Һ�Ը�����ʴ��Ӱ�졣
��1������ֽ��3.5%��NaCl��Һ��ʪ��Ϳ�����ۡ�̼�۵Ļ������ڱ������ϡ�����ֽ�ϼӼ��μ����Լ����ٻ�������NaCl��Һ��û����ֽ������������ʾ��

��ʵ����������˵�����õ��ӵ�������_______________________________��
��̼�۵�������___________________________________________________��
��Ϊ��˵��NaCl�����ã���Ҫ����Ķ���ʵ����_____________________��
��2������ͼʾװ�õ��ձ�a��b�и�����30mL 3.5%��NaCl��Һ���պ�K��ָ��δ����ƫת�������ձ�a��ָ������ƫת��

��ȡa��b����Һ�������μ�K3[Fe(CN)6]��Һ��a�г�����ɫ������b���ޱ仯��b����Ƭ��________����
�ڼ��Ⱥ�ָ�뷢��ƫת��ԭ�������_____________________��
��3���ã�2����ͼʾװ��̽����ͬŨ��NaCl��Һ�Ը�����ʴ��Ӱ�죬���ձ�a��b�и�����30mL��ͬ����������NaCl��Һ��ʵ���¼���±���ʾ��
ʵ�� | a | b | ָ��ƫת���� |
I | 0.1% | 0.01% | ���� |
II | 0.1% | 3.5% | ���� |
�� | 3.5% | ������Һ | ���� |
�����У�b�е缫�����ĵ缫��Ӧʽ��_______________________________��
�����У����ڱ���NaCl��Һ�в��ױ���ʴ���������Ͽ�֪���ڱ���NaCl��Һ��O2Ũ�Ƚϵͣ��������ױ���ʴ�����ʵ��֤����_______________________________��
��4����������ʵ�飬�Ը�����ʴ��Ӱ���������_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���� NA Ϊ�����ӵ�����������˵����ȷ����
A. ��״���£�11.2L CCl4 �к� C��Cl ������Ŀ 1.5NA
B. 8.7g MnO2 �� 40 mL 10 mol��L-1��Ũ�����ַ�Ӧ�����ɵ�����������Ϊ 0.1 NA
C. 1mol NaHSO4�����к��е���������Ϊ 2 NA
D. 0.5mol��L-1K2SO4 ��Һ�У�������������Ϊ 1.5 NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ø�Ũ��������100 mL 1 mol/L��ϡ���ᡣ�ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ�����ƿ�����ձ�����ҩ�ף�����Ͳ����������ƽ����ش��������⣺
(1)����ϡ����ʱ�����������в���Ҫʹ�õ���__________������ţ�����ȱ�ٵ�������____________________��д�������ƣ���
(2)�����㣬����100mL1mol/L��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ____________________________________________mL������һλС��������ȡŨ����ʱӦѡ��_________��ѡ��10mL��50mL ��100mL��������Ͳ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ָ����Ӧ�����ӷ���ʽ��ȷ����
A. ��������ϡNaOH��Һ����Cl2��Cl2+2OH![]() ClO+Cl+H2O
ClO+Cl+H2O
B. �����ۺ�NaOH��Һ��Ӧ��ȡ����H2��Al+2OH![]()
![]() +H2��
+H2��
C. ��������ϡHNO3�ܽ�ͭ��Cu+2![]() +2H+
+2H+![]() Cu2++2NO2��+H2O
Cu2++2NO2��+H2O
D. ��Na2SiO3��Һ�еμ�ϡ���Na2SiO3+2H+![]() H2SiO3��+2Na+
H2SiO3��+2Na+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ȼ�����Һ�����������������ȱ��֢��ҩ�ijҽ��ʵ��С����Ҫ�õ�480 mL���ʵ���Ũ��Ϊ0.5 mol��L��1���Ȼ�����Һ���ش��������⣺
��1�����Ƹ���Һ��������Ȼ��ؾ����������________��
��2�����������Ȼ�����Һ����Ҫʹ�õIJ�����������Ͳ���ձ�����������________��________��
��3����ͼ���ʾ10 mL��Ͳ��Һ���λ�ã��̶�A��B��B��C������1 mL������̶�AΪ9������Ͳ��Һ��������________mL��

��4������ʱ��ijͬѧ����ʾ��ͼ��ͼ����ʾ�����������Ȼ�����Һ��Ũ��________(����ڡ������ڡ���С�ڡ�)0.5 mol��L��1��ijͬѧ���ڲ�������������ʱ����Һ����ڿ̶��ߣ��������ý�ͷ�ιܽ�Һ��������ʹҺ��ǡ�ôﵽ�̶��ߣ��������۸�ͬѧ��������__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��һ��ǰ8���ҹ��������Ƶ���������������DZˮ���ɹ�����������DZӦ�ã����־���ҹ���������DZˮ�������ѽ����Ƚ���������֮�С�

������DZˮ����ѹ��ʹ�õ��������Ͻ���ϡ����в����������Ͻ����ʵ���_______������ĸ����
a���ܶȴ� b��Ӳ�ȴ� c������ʴ
������DZˮ��ͨ�Ŵ���ϵͳ�в���PC(��̼����)��PC�Ͻ��ģ�PC����_______(����ĸ)��
a. �������ϡ���������b. ���ǽ������ϡ���������c. �л��߷��Ӳ���
������DZˮ���еĹܵ�ͨ�����ܽ�Ϊԭ���������ܽ���Ҫ������ϩ���۱�ϩ�����ڡ����������ڡ�������ϩ�ȣ��۱�ϩ����______���ϣ������ȹ���������������������
��2��Ӫ��ƽ�⣬������ҩ�DZ�֤���彡����������������Ҫ;����
����������ʳ���л�ȡ����Ӫ��Ԫ�غ�ά���ء��ơ��������в�������Ԫ�ص���_____����Ԫ�ط��ţ��������߲˸���ά����C���߲����Ա����ʱά����C����ʧС��ԭ����____��
�������������֬�������ʣ�������ø�Ĵ������·���ˮ�ⷴӦ����֬ˮ������ղ����Ǹ�֬�����_________�������ƣ�����������ȫˮ��IJ���A��һ�����еĹ�����Ϊ��COOH��___________���û�ѧ������գ���Ԥ��A�����еĻ�ѧ����Ϊ__________��
a��������������Һ��Ӧ b������ˮ�ⷴӦ c���������ķ�Ӧ
��������̼���������ʹ���������Ը�ð�����Ƹò�Ӧ�ø����˷���______ҩƷ������ĸ����
a����Ƽ� b������ҩ c����˾ƥ��
��Mg2Si3O8��nH2O(������þ)��������θ������������д������θ�ᷴӦ����SiO2��H2O�����ʵĻ�ѧ����ʽ_______
��3����ʮ�������滮��Ҫ��ȷ��������������ǻ���Դϵͳ�����п��ƴ�ͳ��Դ�������ͺ���ʹ����Ϊ��Ҫ��
����ȼú�м���____�ɼ��������е�SO2�ĺ�����ȼú������SO2��NH3�������������ֻ�����Ʒ���ð�ˮ����SO2���Ƶ���������泥�NH4HSO3��д���÷�Ӧ�Ļ�ѧ����ʽ_______
����Ȼˮ�����ʽ϶࣬����Ҫ����������ClO2�����ʴ�����������á�����ClO2��������____
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����仯�����ڹ�ũҵ�����о��й㷺Ӧ�á�
��ش��������⣺
��1����̬Bԭ�ӵļ۵����Ų�ͼ___��B����Ԫ�����ڱ��е�___��Ԫ�ء�
��2��NaBO2������֯��Ư�ס�
�ٵڶ������е�һ�����ܽ���B��O֮���Ԫ��Ϊ___����Ԫ�ط��ţ���
��BO2-�Ŀռ乹��Ϊ____��д���������以Ϊ�ȵ�����ķ��ӵĻ�ѧʽ��____��
��3��BF3��F-�����γ�BF4-��BF3��BF4-��Bԭ�ӵ��ӻ���ʽ�ֱ�Ϊ_____��____��
��4������BN������AlN��Ϊԭ�Ӿ��壬�ṹ���ƣ�BN���۵����AlN��ԭ��Ϊ______��
��5��һ���������ʴ�����ϵľ����ṹ��ͼ��ʾ��
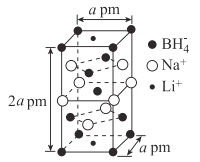
�ٸû�����Ļ�ѧʽΪ____��
���谢���ӵ�������ֵΪNA���þ�����ܶ�Ϊ___g��cm-3���ú�a��NA�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��H2S��CO2�ڸ����·�����Ӧ��CO2 (g) + H2S (g) ![]() COS (g) + H2O (g)����610 Kʱ����0.10 mol CO2��0.40 mol H2S����2.5 L�Ŀո�ƿ������Ӧƽ���ˮ�����ʵ�������Ϊ0.02������˵������ȷ����
COS (g) + H2O (g)����610 Kʱ����0.10 mol CO2��0.40 mol H2S����2.5 L�Ŀո�ƿ������Ӧƽ���ˮ�����ʵ�������Ϊ0.02������˵������ȷ����
A. �����¶ȣ�H2SŨ�����ӣ������÷�Ӧ�Ƿ��ȷ�Ӧ
B. ͨ��CO2������Ӧ������������С
C. H2S��ƽ��ת������ = 4.5 %
D. ��Ӧƽ�ⳣ��K = 2.8��10-3
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com