
��ҵ�϶Ժ�ˮ��Դ�ۺϿ������õIJ��ֹ�����������ͼ��ʾ��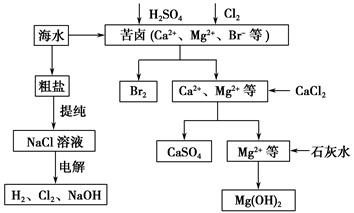
(1)��ⱥ��ʳ��ˮ��������Ĥ���ۺ�Ĥ���ۡ�����Ĥ��Ĥ������ͨ���ķ��ӻ�������________�������е���������Ϊ____________��
(2)�������������Ⱥ��Ƶ�Br2��CaSO4��Mg(OH)2���ܷ�Br2��Mg(OH)2��CaSO4��˳���Ʊ���__________��ԭ����______________________________
(3)�嵥�������Ȼ�̼�е��ܽ�ȱ���ˮ�д�ö࣬���Ȼ�̼��ˮ�����ܣ��ʿ�������ȡ�壬��������������ȴ�������Ȼ�̼��ԭ����________________

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����й���Դ�Ŀ�������˵����ȷ���� �� ��
| A���Ӻ�������ȡ�ⵥ�ʵĹ���һ���漰������ԭ��Ӧ |
| B���Ӻ�ˮ�п��Եõ�NaCl�����NaCl��Һ���Ʊ�Na��Cl2 |
| C����Ȼ�����Ҵ���ˮú���ֱ����ڻ�ʯ��Դ������������Դ�Ͷ�����Դ |
| D��Cu��Al��Hg���Էֱ����Ȼ�ԭCuO�����AlCl3���ȷֽ�HgOұ���õ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��������ҵ�Դٽ����ú���ᷢչ������Ҫ���á�
�ٲ���ֺ��е�CrԪ���������ֹ��̵����� ���ǰ�������롣
������ʱ������衢�̺�����Ŀ���� ��
�����������������У�β�������е���Ҫ��Ⱦ���� ���ӻ����;��ýǶȿ��ǣ�����β�������������� ��
��2��������һ����Ҫ�Ļ���ԭ�ϡ�Ŀǰ�Ƽҵ��Ҫ�С�������͡������Ƽ�����ֹ��ա�
�١��������������CaCl2�����д���ù����в���CaCl2�Ļ�ѧ����ʽ�� ��
��д���������Ƽ���йط�Ӧ�Ļ�ѧ����ʽ�� ��
��CO2���Ƽҵ����Ҫԭ�ϣ��������Ƽ���롰�������CO2����Դ�кβ�ͬ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����Ǹ������Դ���⣬��ˮ��Դ�����þ��зdz������ķ�չǰ������ˮ����Ԫ����Br����ʽ���ڣ���ҵ���ÿ����������Ӻ�ˮ����ȡ��Ĺ�������������ʾ��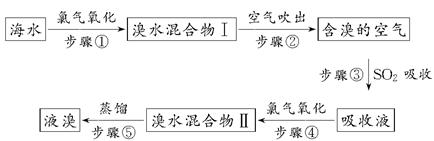
(1)����ٷ�Ӧ�����ӷ���ʽΪ_______________________________��
(2)����۷�Ӧ�Ļ�ѧ����ʽΪ_________________________________��
(3)Br��ԭ��������________�������ڱ���λ�ڵ�________���ڡ�________�塣
(4)���������Ĺ����У��¶�Ӧ������80��90�档�¶ȹ�����Ͷ������������������ԭ��________________��
(5)Ϊʲô��ֱ���á���ˮ������Ҫ�á���ˮ�����������ó�Һ�壿
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ݿ�ѧ��Ԥ�⣺�ٹ�100���ȫ�����¹��ƽ�������Լ1.4��5.8 �档������һԤ�⣬ȫ��������������ȫ���������ɹ����Ӱ�죬����ˮ��Դ���ѷ���������塣��ˮռ�����ܴ�ˮ����97.2%�����Ѻ�ˮ�����ͻ�������������������ܽ����ˮ��Դȱ�������⣬���ܳ�����ú�����Դ��
��1����Ŀǰ�������ԣ������Դ���ĵ����⣬���������ڡ���ˮ�������ļ����� ������ţ���
| A������ | B������������ | C����ᷨ�� | D�����ӽ�������E�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ۺ����ü������ڽ�Լ��Դ���������ڱ���������ʵ�������÷Ͼɻ�ͭ(Cu��Zn�Ͻ𣬺���������Fe)�Ʊ���������(CuSO4��5H2O)��������ZnO���Ʊ�����ͼ���£� 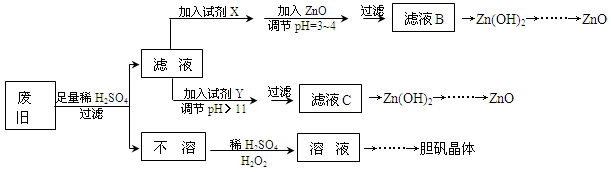
��֪��Zn���������������Al����������������ƣ�pH��11ʱZn(OH)2������NaOH��Һ����[Zn(OH)4]2�����±��г��˼������������������������pH(��ʼ������pH����������Ũ��Ϊ1.0mol��L��1����)��
| | Fe3�� | Fe2�� | Zn2�� |
| ��ʼ������pH | 1.1 | 5.8 | 5.9 |
| ������ȫ��pH | 3.0 | 8.8 | 8.9 |
 2CuI(��ɫ)����I2��I2��2S2O32��
2CuI(��ɫ)����I2��I2��2S2O32�� 2I����S4O62��
2I����S4O62���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����þ����Ҫ�ɷ�ΪMgCO3������ȼ��������þ�Ĺ����������£�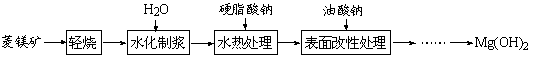
��1����������ͼ���Եó��Ľ���Ϊ �� ��
ͼ1 25��ʱMgOˮ����ʱ��仯X����������ͼ 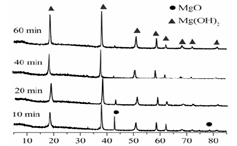
ͼ2 90��ʱMgOˮ����ʱ��仯X����������ͼ
��2��ˮ����ӦMgO+H2O = Mg(OH)2���Է����е�ԭ���� ��
��3�����Ԫ�������ɺͱ�1��֪�����������������ȷֽ�Ĺ����� ����дһ�����ɣ�
��1 ��������Ԫ�صĽ������������ȷֽ��¶�/��
| LiOH | NaOH | KOH | Al(OH)3 | Mg(OH)2 | Ca(OH)2 | Ba(OH)2 |
| 924 | ���ֽ� | ���ֽ� | 140 | 258 | 390 | 700 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ����������������Fe2+��Fe3+�������μ�����CaO��MgO���Ʊ��ߵ���������(Fe2O3 )�ͻ���(NH4)2SO4�����������������£�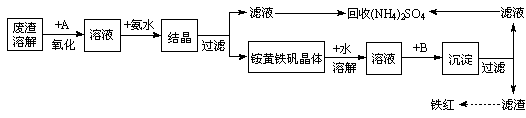
�ش��������⣺
��1���ڷ����ܽ����ʱ��Ϊ�˼��ٷ����ܽ�Ĵ�ʩ�ǣ�___________________��__________________����д���㣩
��2������A��һ��������
�ٹ�ҵ�����ѡ�� ������ţ�
| A������ | B��Cl2 | C��MnO2 | D��H2O2�� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������ʮ�����Ӻ�ˮ��Դ���ۺ����á����в���Ҫ��ѧ�仯���ܹ��Ӻ�ˮ�л�õ�������( )
| A���ȡ��塢�� | B��ʳ�Ρ���ˮ | C���ռ���� | D���ơ�þ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com